In order to apply the physical models described in earlier sections to the prediction of the discrete phase trajectories and heat/mass transfer, Ansys Fluent requires many physical property inputs.
For additional information, see the following sections:
Table 24.1: Property Inputs for Inert Particles – Table 24.5: Property Inputs for Multicomponent Particles (Law 7) summarize which of these property inputs are used for each particle type and in which of the equations for heat and mass transfer each property input is used. Detailed descriptions of each input are provided in Setting Discrete-Phase Physical Properties.
Table 24.1: Property Inputs for Inert Particles
| Property | Symbol |
|---|---|
| density |
|
| specific heat |
|
| thermal conductivity |
|
| particle emissivity |
|
| particle scattering factor |
|
| thermophoretic coefficient |
|
Table 24.2: Property Inputs for Droplet Particles
| Properties | Symbol |
|---|---|
| density |
|
| specific heat |
|
| thermal conductivity |
|
| viscosity |
|
| latent heat |
|
| vaporization temperature |
|
| boiling point |
|
| volatile component fraction |
|
| binary diffusivity |
|
| diffusivity reference pressure |
|
| saturation vapor pressure |
|
| heat of pyrolysis |
|
| droplet surface tension |
|
| particle emissivity |
|
| particle scattering factor |
|
| composition averaging coefficient |
|
| temperature averaging coefficient |
|
| Thermolysis model | |
| – single-rate | |
| pre-exponential factor |
|
| activation energy |
|
| – constant | |
| rate constant |
|
Table 24.3: Property Inputs for Combusting Particles (Laws 1–4)
| Property | Symbol |
|---|---|
| density |
|
| specific heat |
|
| thermal conductivity |
|
| latent heat |
|
| vaporization temperature |
|
| volatile component fraction |
|
| swelling coefficient |
|
| burnout stoichiometric ratio |
|
| combustible fraction |
|
| heat of reaction for burnout |
|
| fraction of reaction heat given to solid |
|
| particle emissivity |
|
| particle scattering factor |
|
| thermophoretic coefficient |
|
| devolatilization model | |
| – law 4, constant rate | |
| – – constant |
|
| – law 4, single rate | |
| – – pre-exponential factor |
|
| – – activation energy |
|
| – law 4, two rates | |
| – – pre-exponential factors |
|
| – – activation energies |
|
| – – weighting factors |
|
| – law 4, CPD | |
| – – initial fraction of bridges in coal lattice |
|
| – – initial fraction of char bridges |
|
| – – lattice coordination number |
|
| – – cluster molecular weight |
|
|
– – side chain molecular weight |
|
Table 24.4: Property Inputs for Combusting Particles (Law 5)
| Property | Symbol |
|---|---|
| combustion model | |
| – law 5, diffusion rate | |
| – – binary diffusivity |
|
| – – diffusivity reference pressure |
|
| – law 5, diffusion/kinetic rate | |
| – – mass diffusion limited rate constant |
|
| – – kinetics limited rate pre-exp. factor |
|
| – – kinetics limited rate activation energy |
|
| – law 5, intrinsic rate | |
| – – mass diffusion limited rate constant |
|
| – – kinetics limited rate pre-exp. factor |
|
| – – kinetics limited rate active energy |
|
| – – char porosity |
|
| – – mean pore radius |
|
| – – specific internal surface area |
|
| – – tortuosity |
|
| – – burning mode |
|
Table 24.5: Property Inputs for Multicomponent Particles (Law 7)
| Property | Symbol |
|---|---|
| mixture species | selected droplets for components |
| density |
|
| specific heat |
|
| thermal conductivity |
|
| vapor particle equilibrium |
|
| thermophoretic coefficient |
|
| composition averaging coefficient |
|
| temperature averaging coefficient |
|
When you create a particle injection and define the initial conditions for the discrete phase (as described in Setting Initial Conditions for the Discrete Phase), you choose a particular material as the particle’s material. All particle streams of that material will have the same physical properties.
Important: Note that you will not choose a Material for a Massless particle type in the Set Injections Properties dialog box.
Discrete-phase materials are divided into four categories, corresponding to the four types of particles available. These material types are:
inert-particle
droplet-particle
combusting-particle
multicomponent-particle
Each material type will be added to the Material Type list in the Create/Edit Materials Dialog Box when an injection of that type of particle is defined (in the Set Injection Properties or Set Multiple Injection Properties dialog box, as described in Setting Initial Conditions for the Discrete Phase). The first time you create an injection of each particle type, you will be able to choose a material from the database, and this will become the default material for that type of particle. That is, if you create another injection of the same type of particle, your selected material will be used for that injection as well. You may choose to modify the predefined properties for your selected particle material, if you want (as described in Modifying Properties of an Existing Material). If you need only one set of properties for each type of particle, you need not define any new materials; you can simply use the same material for all particles.
Important:
If you do not find the material you want in the database, you can select a material that is close to the one you want to use, and then modify the properties and give the material a new name, as described in Creating a New Material.
Note that a discrete-phase material type will not appear in the Material Type list in the Create/Edit Materials dialog boxes until you have defined an injection of that type of particles. This means, for example, that you cannot define or modify any combusting-particle materials until you have defined a combusting particle injection (as described in Setting Initial Conditions for the Discrete Phase).
For a particle-mixture material type, you must select the species in your mixture. To do this:
Click the button next to Mixture Species in the Create/Edit Materials dialog box (Properties group box).
In the Species dialog box that opens, add your species to the Selected Species list.
Select Multicomponent in the Set Injection Properties dialog box (Particle Type group box).
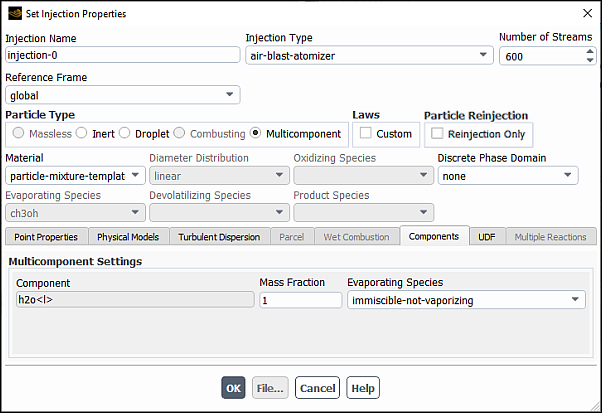
The selected species will now be available, under the Components tab (Figure 24.55: The Components Tab).
In many cases, a single set of physical properties (density, heat capacity, and so on) is appropriate for each type of discrete phase particle considered in a given model. Sometimes, however, a single model may contain two different types of inert, droplet, combusting particles, or multicomponent particles (for example, heavy particles and gaseous bubbles or two different types of evaporating liquid droplets). In such cases, it is necessary to assign a different set of properties to the two (or more) different types of particles. This is easily accomplished by defining two or more inert, droplet, or combusting particle materials and using the appropriate one for each particle injection.
You can define additional discrete-phase materials either by copying them from the database or by creating them from scratch. See Using the Create/Edit Materials Dialog Box for instructions on using the Create/Edit Materials Dialog Box to perform these actions.
Important: Recall that you must define at least one injection (as described in Setting Initial Conditions for the Discrete Phase) containing particles of a certain type before you will be able to define additional materials for that particle type.
The properties that appear in the Create/Edit Materials Dialog Box vary depending on the particle type (selected in the Set Injection Properties or Set Multiple Injection Properties dialog box, as described in Defining Injection Properties and Defining Properties Common to More than One Injection) and the physical models you are using in conjunction with the discrete-phase model.
All properties you may need to define for a discrete-phase material are listed below (alphabetically). See Table 24.1: Property Inputs for Inert Particles – Table 24.4: Property Inputs for Combusting Particles (Law 5) to see which properties are defined for each type of particle.
- Binary Diffusivity
is the mass diffusion coefficient used in the vaporization law (Law 2),
in Equation 12–83 and
in Equation 12–276 in the Theory Guide. This input is also used to define the mass diffusion of the oxidizing species to the surface of a combusting particle,
, as given in Equation 12–146 in the Theory Guide. (Note that the diffusion coefficient inputs that you supply for the continuous phase are not used for the discrete phase.)
For Droplet Particle type Materials, select film-averaged from the Binary Diffusivity drop-down list and specify parameters for the film-averaged model (see Equation 12–89 in the Theory Guide) using the Film Binary Diffusivity dialog box. The film-averaged model is recommended when accurate temperature-dependent binary diffusivity data are available.
To apply the unity Lewis number model (Equation 12–90 in the Fluent Theory Guide) select unity-lewis-number from the Binary Diffusivity drop-down. The unity Lewis number model is a simplified approach and is not appropriate when the molecular weights of the evaporating species (or oxidizing species for combusting particles) and the gas-phase mixture are very different. The model can be applied, for example, for the evaporation of water, light hydrocarbons, or methanol in air, but is not appropriate for heavy hydrocarbons.
You also have the option of implementing a user-defined function to model the particle binary diffusivity. See
DEFINE_DPM_PROPERTYin the Fluent Customization Manual for more information.To enable the pressure dependence for the binary diffusivity, in the Create/Edit Materials dialog box, under Properties, from the Diffusivity Reference Pressure drop-down list, select constant (see Equation 12–91 in the Fluent Theory Guide) and enter a value for the reference pressure
that corresponds to the value defined for the Binary Diffusivity material property. The pressure dependence option is available with all property methods for the binary diffusivity, including user-defined, with the exception of the unity Lewis number model, for which the pressure dependence is already accounted for through the density variable in the model equation.
- Boiling Point
is the temperature,
, at which the calculation of the boiling rate equation (Equation 12–101 in the Theory Guide) is initiated by Ansys Fluent. When a droplet particle reaches the boiling point, Ansys Fluent applies Law 3 and assumes that the droplet temperature is constant at
. The boiling point denotes the temperature at which the particle law transitions from the vaporization law to the boiling law.
For multicomponent particles the boiling point of the components is used only as a reference temperature of the latent heat. Instead, the boiling starts when the sum of the partial component saturation pressures reach the total fluid pressure. The definition of the saturation pressure curve is therefore essential for the boiling of multicomponent particles.
You also have the option of implementing a user-defined function to model the particle boiling point. See
DEFINE_DPM_PROPERTYin the Fluent Customization Manual for more information.Further information can be found in Defining the Boiling Point and Latent Heat in the Fluent Theory Guide and Considering Pressure Dependence in Boiling.
- Burnout Stoichiometric Ratio
is the stoichiometric requirement,
, for the burnout reaction, Equation 12–145 in the Theory Guide, in terms of mass of oxidant per mass of char in the particle.
- Combustible Fraction
is the mass fraction of char,
, in the coal particle, that is, the fraction of the initial combusting particle that will react in the surface reaction, Law 5 (Equation 12–144 in the Theory Guide).
- Combustion Model
defines which version of the surface char combustion law (Law5) is being used. You can choose from the following options:
diffusion-limited (default)
The binary diffusivity defined above will be used in Equation 12–146 in the Theory Guide.
kinetics/diffusion-limited
When the kinetics/diffusion-limited limited rate model is selected for the surface combustion model, the Kinetics/Diffusion-Limited Combustion Model Dialog Box opens where you can enter the Mass Diffusion Limited Rate Constant (
in Equation 12–147 in the Theory Guide), Kinetics Limited Rate Pre-exponential Factor (
in Equation 12–148), and Kinetics Limited Rate Activation Energy (
in Equation 12–148).
Note that the Kinetics/Diffusion-Limited Combustion Model dialog box is a modal dialog box, which means that you must tend to it immediately before continuing the property definitions.
intrinsic-model
When the intrinsic-model is selected for the surface combustion model, the Intrinsic Combustion Model Dialog Box opens where you can enter the Mass Diffusion Limited Rate Constant (
in Equation 12–147 in the Theory Guide), Kinetics Limited Rate Pre-exponential Factor (
in Equation 12–157), Kinetics Limited Rate Activation Energy (
in Equation 12–157), Char Porosity (
in Equation 12–154), Mean Pore Radius (
in Equation 12–156), Specific Internal Surface Area (
in Equation 12–151 Equation 12–153), Tortuosity (
in Equation 12–154), and Burning Mode, alpha (
in Equation 12–158).
Note that the Intrinsic Combustion Model dialog box is a modal dialog box, which means that you must tend to it immediately before continuing the property definitions.
multiple-surface-reactions
When multiple-surface-reactions is selected, Ansys Fluent displays the Multiple Surface Reactions Dialog Box informing you to open the Reactions dialog box, where you can review or modify the particle surface reactions that you specified as described in Overview of User Inputs for Modeling Species Transport and Reactions. In addition, you can set property options for the char specific heat and density:
- Composition Dependent Specific Heat
if this option is enabled, your input for the particle
property will be used to determine the specific heat of the volatiles and ash component only. The specific heat of the char will be calculated as a mass-weighted average of the particle surface species specific heat values, and the particle specific heat is finally calculated as the mass average of the char and volatiles+ash fractions. The specific heat of the particle surface species should be defined in the corresponding fluid materials of the Mixture material.
- Composition Dependent Density
if this option is enabled, your input for the particle Density property will be used to determine the density of the volatiles and ash component. The density of the char will be calculated as a volume-weighted average value of the particle surface species densities, and the particle density is finally calculated as the volume-weighted average of the char and volatiles+ash fractions. The density of the particle surface species should be defined in the corresponding fluid materials of the Mixture material.
Important: If you have not yet defined any particle surface reactions, you must be sure to define them now. See Using the Multiple Surface Reactions Model for Discrete-Phase Particle Combustion for more information about using the multiple surface reactions model.
You will notice that the Burnout Stoichiometric Ratio and Heat of Reaction for Burnout are no longer available in the Create/Edit Materials dialog box, as these parameters are now computed from the particle surface reactions you defined in the Reactions dialog box.
Note that the multiple surface reactions model is available only if the Particle option for Reactions is enabled in the Species Model dialog box. See User Inputs for Particle Surface Reactions for details.
- Composition Averaging Coefficient
is the coefficient
in Equation 12–186 in the Fluent Theory Guide. To assume bulk gas composition for the physical properties in the particle vaporization and heating rates equations, select none from the Composition Averaging Coefficient drop-down in the Create/Edit Materials dialog box, under Properties. To enable property averaging, select constant and enter a value between 0 and 1 for the Composition Averaging Coefficient.
= 1 corresponds to bulk gas mixture. For
= 0, the composition reverts to the particle surface composition.
Important: When you define the Composition Averaging Coefficient with a constant value other than 1, you must also specify Specific Heat, Viscosity and Thermal Conductivity for the evaporating species (in the Create/Edit Materials dialog box, under Properties, for the corresponding fluid material of the continuous phase mixture). Note that for the definition of the evaporating species properties, the user-defined method is not supported and should not be used.
The default and recommended value for the averaging coefficient is 1/3. However, to improve the simulation results, you must provide accurate temperature-dependent data for the vapor material (through the Create/Edit Materials dialog box).
- Cp
is the specific heat,
, of the particle. The specific heat may be defined as a function of temperature by selecting one of the function types from the drop-down list to the right of Cp. See Defining Properties Using Temperature-Dependent Functions for details about temperature-dependent properties. For multicomponent particles, it can be calculated as a mass-weighted value of the specific heat of the droplet component.
You also have the option of implementing a user-defined function to model the particle specific heat. See
DEFINE_DPM_PROPERTYin the Fluent Customization Manual for more information.A composition-dependent char specific heat option can be enabled if you are using the multiple-surface-reactions model for a combusting particle. For details on enabling this model, see Combustion Model .
When you are using the non-premixed or the partially-premixed combustion model in the continuous phase calculation, the specific heat defined for the particle material will be used for the specific heat and enthalpy calculations of the non-volatile/non-reacting particle mass.
- Density
is the density of the particulate phase in units of mass per unit volume of the discrete phase. This density is the mass density and not the volumetric density. The density may be defined as a function of temperature by selecting one of the function types from the drop-down list to the right of Density. See Defining Properties Using Temperature-Dependent Functions for details about temperature-dependent properties. For compressible flows, or if any of the real-gas models is enabled, the compressible-liquid method is also available. See Compressible Liquid Density Method for details. For multicomponent particles, it can be calculated as a volume-weighted value of the density of the droplet components.
You also have the option of implementing a user-defined function to model the particle density. See
DEFINE_DPM_PROPERTYin the Fluent Customization Manual for more information.For a combusting particle that swells during the trajectory calculations, the temperature-dependent density calculation is suspended during the devolatilization law and your input is used to determine the initial particle diameter,
at the start of the devolatilization in Equation 12–138 in the Fluent Theory Guide. A composition-dependent char density option can be enabled if you are using the multiple-surface-reactions model for a combusting particle. For details on enabling this model, see Combustion Model .
- Devolatilization Model
defines which version of the devolatilization model, Law 4, is being used.
You can choose the following devolatilization models (as described in Devolatilization (Law 4) in the Theory Guide):
constant (default)
To use the default constant rate devolatilization model (as described in Equation 12–107 in the Theory Guide), enter the rate constant
in the field below the list.
For more information, see The Constant Rate Devolatilization Model in the Fluent Theory Guide.
single rate
When the single kinetic rate model is selected, the Single Rate Model Dialog Box opens where you can enter the Pre-exponential Factor,
, and the Activation Energy,
, to be used in Equation 12–109 in the Theory Guide for the computation of the kinetic rate.
For more information, see The Single Kinetic Rate Model in the Fluent Theory Guide.
two-competing-rates
When the two competing rates model (two-competing-rates) is selected, the Two Competing Rates Model Dialog Box opens where you can enter, for the First Rate and the Second Rate, the Pre-exponential Factor (
in Equation 12–111 and
in Equation 12–112 in the Theory Guide), Activation Energy (
in Equation 12–111 and
in Equation 12–112), and Weighting Factor (
and
in Equation 12–113). The constants you specify are used in Equation 12–111 through Equation 12–113.
For more information, see The Two Competing Rates (Kobayashi) Model in the Fluent Theory Guide.
cpd-model
When the CPD model (cpd-model) is selected, the CPD Model dialog box opens where you can enter the Initial Fraction of Bridges in Coal Lattice (
in Equation 12–124 of the Theory Guide), Initial Fraction of Char Bridges (
in Equation 12–123), Lattice Coordination Number (
in Equation 12–135), Cluster Molecular Weight (
in Equation 12–135), and Side Chain Molecular Weight (
in Equation 12–134).
For more information, see The CPD Model in the Fluent Theory Guide.
Note that the Single Rate Model, Two Competing Rates Model, and CPD Model dialog boxes are modal dialog boxes, which means that you must tend to them immediately before continuing the property definitions.
Note that by default, the minimum volatiles mass fraction is 0.01. You can lower this value by using the following text command:
define/models/dpm/options/lowest-volatiles-mass-fraction- Diffusivity Reference Pressure
is the reference pressure
for the pressure dependent binary diffusivity in Equation 12–91 in the Fluent Theory Guide. To apply the pressure dependence, in the Create/Edit Materials dialog box, under Properties, from the Diffusivity Reference Pressure drop-down list, select constant and enter a reference pressure value corresponding to the value for the Binary Diffusivity material property. If none is selected from the Diffusivity Reference Pressure drop-down list, Ansys Fluent assumes no pressure dependence for the binary diffusivity.
- Heat of Pyrolysis
is the heat of the instantaneous pyrolysis reaction ,
, that the evaporating/boiling species may undergo when released to the continuous phase. This input represents the conversion of the evaporating species to lighter components during the evaporation process. The heat of pyrolysis should be specified as a positive number for exothermic reaction and as a negative number for endothermic reaction. The default value of zero implies that the heat of pyrolysis is not considered. This input is used in Equation 12–508 in the Theory Guide.
- Heat of Reaction for Burnout
is the heat released by the surface char combustion reaction, Law 5 (Equation 12–145 in the Theory Guide). This parameter is specified in terms of heat release (for example, Joules) per unit mass of char consumed in the surface reaction.
- Latent Heat
is the latent heat of vaporization,
, required for phase change from an evaporating liquid droplet (Equation 12–93, Equation 12–283, Equation 12–284, and Equation 12–301 in the Theory Guide) or for the evolution of volatiles from a combusting particle (Equation 12–139 in the Theory Guide). This input is supplied in units of J/kg in SI units or of Btu/
in British units and is treated as a constant by Ansys Fluent. For the droplet particle, the latent heat value at the boiling point temperature should be used.
You also have the option of implementing a user-defined function to model the particle latent heat. See
DEFINE_DPM_PROPERTYin the Fluent Customization Manual for more information.Further information can be found in Defining the Boiling Point and Latent Heat in the Fluent Theory Guide and Including the Effect of Droplet Temperature on Latent Heat.
- React. Heat Fraction Absorbed by Solid
is the parameter
(Equation 12–159 in the Theory Guide), which controls the distribution of the heat of reaction between the particle and the continuous phase. The default value of zero implies that the entire heat of reaction is released to the continuous phase.
- Reaction Model
allows you to select the reaction model for the particle mixture material. You can select either multicomponent-reactions or none if you do not want to model reactions in particle mixture material. See Multicomponent Particles with Chemical Reactions for details. This item appears only for cases with reacting multicomponent particles.
- Saturation Vapor Pressure
is the saturated vapor pressure,
, defined as a function of temperature, which is used in the vaporization law, Law 2 (Equation 12–81 in the Theory Guide). The saturated vapor pressure may be defined as a function of temperature by selecting one of the function types from the drop-down list to the right of its name (see Defining Properties Using Temperature-Dependent Functions for details about temperature-dependent properties). You may also define the saturated vapor pressure using RGP tables as described in Defining Saturation Properties via RGP Tables. In the case of unrealistic inputs, Ansys Fluent restricts the range of
to between 0.0 and the operating pressure. Correct specification of a realistic vapor pressure curve is essential for accurate results from the vaporization model.
You also have the option of implementing a user-defined function to model the particle saturation vapor pressure. See
DEFINE_DPM_PROPERTYin the Fluent Customization Manual for more information.- Swelling Coefficient
is the coefficient
in Equation 12–138 in the Theory Guide, which governs the swelling of the coal particle during the devolatilization law, Law 4 (Devolatilization (Law 4) in the Theory Guide). A swelling coefficient of unity (the default) implies that the coal particle stays at constant diameter during the devolatilization process.
You also have the option of implementing a user-defined function to model the particle swelling coefficient. See
DEFINE_DPM_PROPERTYin the Fluent Customization Manual for more information.- Temperature Averaging Coefficient
is the coefficient
in Equation 12–185 in the Fluent Theory Guide. To assume bulk gas temperature for the physical properties in the particle vaporization and heating rates equations, select none from the Temperature Averaging Coefficient drop-down in the Create/Edit Materials dialog box, under Properties. To enable property averaging, select constant and enter a value between 0 and 1 for the Temperature Averaging Coefficient.
= 1 corresponds to bulk gas conditions. For
= 0, the temperature reverts to the particle surface temperature.
Important: When you define the Temperature Averaging Coefficient with a constant value other than 1, you must also define Specific Heat, Viscosity and Thermal Conductivity for the evaporating species (in the Create/Edit Materials dialog box, under Properties, for the corresponding fluid material of the continuous phase mixture). Note that for the definition of the evaporating species properties, the user-defined method is not supported and should not be used.
The default and recommended value for the averaging coefficient is 1/3. However, to improve the simulation results, you must provide accurate temperature-dependent data for the evaporating vapor material (through the Create/Edit Materials dialog box).
- Thermal Conductivity
is the thermal conductivity of the particle,
. This input is specified in units of W/m-K in SI units or
in British units and is treated as a constant by Ansys Fluent.
- Thermolysis Model
defines which Thermolysis model is used for the calculation of the mass transfer of the vaporizing species from the droplet to the bulk phase according to a Thermolysis rate equation. You can choose from the following options:
single-rate
When single-rate is selected, the Single Rate Model Dialog Box opens where you specify the Pre-exponential Factor
and the Activation Energy
to be used in Equation 12–87 (if you are modeling a droplet material), or the Pre-exponential Factor
and Activation Energy
in Equation 12–164 in the Fluent Theory Guide (if you are modeling multicomponent particles).
secondary-rate
(cases with the Lagrangian Wall Film model only) When secondary-rate is selected, the Secondary Rate Model Dialog Box opens where you specify the Pre-exponential Factor
and Activation Energy
under the Particle Thermolysis Rate and the Pre-exponential Factor
and Activation Energy
under the Film Thermolysis Rate. These values will be used in Equation 12–87 in the Fluent Theory Guide if you are modeling a droplet material and a film or in Equation 12–164 in the Fluent Theory Guide if you are modeling multicomponent particles and a film.
constant
For the constant rate Thermolysis model, enter the rate constant
to be used in Equation 12–88 (if you are modeling a droplet material), or the rate constant
in Equation 12–165 in the Fluent Theory Guide (if you are modeling multicomponent particles).
none (default) signifies that the Thermolysis rate model is disabled.
Note that if you are using a multicomponent particle, the Thermolysis model is set for the individual droplet materials. A typical application is the modeling of Selective Catalytic Reduction (SCR) systems where it is recommended to use the single-rate Thermolysis model for the urea-liquid component in the urea-water particle mixture.
- Thermophoretic Coefficient
is the coefficient
in Equation 12–11 in the Theory Guide, and appears when the thermophoretic force (which is described in Thermophoretic Force in the Theory Guide) is included in the trajectory calculation (that is, when the Thermophoretic Force option is enabled in the Discrete Phase Model dialog box). The default is the expression developed by Talbot [158] (talbot-diffusion-coeff) and requires no input from you. You can also define the thermophoretic coefficient as a function of temperature by selecting one of the function types from the drop-down list to the right of Thermophoretic Coefficient. See Defining Properties Using Temperature-Dependent Functions for details about temperature-dependent properties.
You also have the option of implementing a user-defined function to model the particle thermophoretic coefficient. See
DEFINE_DPM_PROPERTYin the Fluent Customization Manual for more information.- Vaporization Temperature
is the temperature,
, at which the calculation of vaporization from a liquid droplet or devolatilization from a combusting particle is initiated by Ansys Fluent. Until the particle temperature reaches
, the particle is heated via Law 1, Equation 12–70 in the Theory Guide. This temperature input represents a modeling decision rather than any physical characteristic of the discrete phase.
You also have the option of implementing a user-defined function to model the particle vaporization temperature. See
DEFINE_DPM_PROPERTYin the Fluent Customization Manual for more information.- Vapor-Particle-Equilibrium
is the selected approach for the calculation of the vapor concentration of the components at the surface. This can be Raoult’s law (Equation 12–176 in the Theory Guide), the Peng-Robinson real gas model (Equation 12–184 in the Theory Guide), or a user-defined function that defines the equilibrium.
- Vaporization/Boiling Model
defines which vaporization/boiling model is used for pure droplets (Law 2) and for multicomponent droplets (Law 7). You can choose from the following options:
diffusion-controlled (default)
This option will apply Equation 12–80 in the Theory Guide.
convection/diffusion-controlled
When you select the convection/diffusion-controlled model for vaporization, Equation 12–85 in the Theory Guide will be applied for the calculation of the vaporization rate, and Equation 12–97 in the Theory Guide will be applied in the particle heat transfer calculations. This model is recommended when evaporation rates are high. For slowly evaporating droplets both models are expected to give similar results.
Once you have selected convection/diffusion-controlled, you can enable the Variable Lewis Number Formulation option in the Model Options dialog box to compute the Spalding heat number from Equation 12–96 in the Fluent Theory Guide. If the option is disabled Ansys Fluent uses Equation 12–97.
You can also enable averaging of the Spalding heat transfer term for the convection/diffusion-controlled model according to Equation 12–98 in the Fluent Theory Guide by using the TUI command
define/models/dpm/options/vaporization-heat-transfer-averaging. This option has the net effect of increasing the heat-transfer to the droplet and may produce more realistic results for cases with large temperature differences between droplet and bulk gas.
For both diffusion-controlled and convection/diffusion-controlled models, you can select Use the Specific Heat of the Evaporating Species in Boiling Law in the Model Options Dialog Box. This option sets the heat capacity of the gas (
in Equation 12–101 in the Fluent Theory Guide) to the specific heat of the evaporating species selected in the Set Injection Properties dialog box. For the convection/diffusion controlled model with the Variable Lewis Number Formulation, this option is selected by default. This ensures consistency with the convection/diffusion controlled model expression for
in Equation 12–95 in the Fluent Theory Guide.
- Volatile Component Fraction
(
) is the mass fraction of a droplet particle that may vaporize via Laws 2 and/or 3 (Droplet Vaporization (Law 2) and Droplet Boiling (Law 3) in the Fluent Theory Guide). For combusting particles, it is the mass fraction of volatiles that may be evolved via Law 4 (Devolatilization (Law 4) in the Theory Guide).
Note that the non-volatile fraction in the droplet is assumed to be immiscible with the evaporating mass fraction.
When the effect of particles on radiation is enabled (for the P-1 or discrete ordinates radiation model only) in the Discrete Phase Model Dialog Box, you must define the following additional parameters:
- Particle Emissivity
is the emissivity of particles in your model,
, used to compute radiation heat transfer to the particles (Equation 12–70, Equation 12–93, Equation 12–104, Equation 12–139, and Equation 12–159 in the Theory Guide) when the P-1 or discrete ordinates radiation model is active. Note that you must enable radiation to particles, using the Particle Radiation Interaction option in the Discrete Phase Model Dialog Box. Recommended values of particle emissivity are 1.0 for coal particles and 0.5 for ash [92].
You also have the option of implementing a user-defined function to model the particle emissivity. See
DEFINE_DPM_PROPERTYin the Fluent Customization Manual for more information.- Particle Scattering Factor
is the scattering factor,
, due to particles in the P-1 or discrete ordinates radiation model (Equation 5–73 in the Theory Guide). Note that you must enable particle effects in the radiation model, using the Particle Radiation Interaction option in the Discrete Phase Model Dialog Box. The recommended value of
for coal combustion modeling is 0.9 [92]. Note that if the effect of particles on radiation is enabled, scattering in the continuous phase will be ignored in the radiation model.
You also have the option of implementing a user-defined function to model the particle scattering factor. See
DEFINE_DPM_PROPERTYin the Fluent Customization Manual for more information.
When an atomizer injection model and/or the droplet breakup or collision model is enabled in the Set Injection Properties Dialog Box (atomizers) and/or Discrete Phase Model Dialog Box (droplet breakup/collision), you must define the following additional parameters:
- Droplet Surface Tension
is the droplet surface tension,
. The surface tension may be defined as a function of temperature by selecting one of the function types from the drop-down list to the right of Droplet Surface Tension. See Defining Properties Using Temperature-Dependent Functions for details about temperature-dependent properties. You also have the option of implementing a user-defined function to model the droplet surface tension. More information about user-defined functions can be found in the Fluent Customization Manual.
- Viscosity
is the droplet viscosity,
. The viscosity may be defined as a function of temperature by selecting one of the function types from the drop-down list to the right of Viscosity. See Defining Properties Using Temperature-Dependent Functions for details about temperature-dependent properties. You also have the option of implementing a user-defined function to model the droplet viscosity. More information about user-defined functions can be found in the Fluent Customization Manual.