Theoretical information about particle reactions is presented in the following sections:
As described in The Multiple Surface Reactions Model, it is possible to define multiple particle surface reactions to model the surface combustion of a combusting discrete-phase particle. This section provides theoretical background about particle surface reactions. Information can be found in the following sections:
- 7.1.3.1.1. General Description
- 7.1.3.1.2. Ansys Fluent Model Formulation
- 7.1.3.1.3. Extension for Stoichiometries with Multiple Gas Phase Reactants
- 7.1.3.1.4. Solid-Solid Reactions
- 7.1.3.1.5. Solid Decomposition Reactions
- 7.1.3.1.6. Solid Deposition Reactions
- 7.1.3.1.7. Gaseous Solid Catalyzed Reactions on the Particle Surface
For more information about using particle surface reactions, see Combusting Particle Surface Reactions in the User's Guide.
The relationships for calculating char particle burning rates are presented
and discussed in detail by Smith [609]. The particle
reaction rate, (
-s), can be expressed as
(7–98) |
| where, | |
|
| |
|
| |
|
| |
|
| |
|
|
In Equation 7–98, the concentration at the particle
surface, , is not known, so it should be eliminated, and the expression
is recast as follows:
(7–99) |
This equation has to be solved by an iterative procedure, with the exception
of the cases when or
. When
, Equation 7–99 can be written
as
(7–100) |
In the case of , if there is a finite concentration of reactant at the
particle surface, the solid depletion rate is equal to the chemical reaction
rate. If there is no reactant at the surface, the solid depletion rate changes
abruptly to the diffusion-controlled rate. In this case, however, Ansys Fluent will
always use the chemical reaction rate for stability reasons.
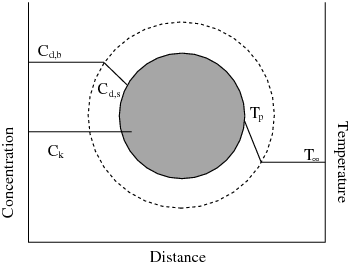
A particle undergoing an exothermic reaction in the gas phase is shown
schematically in Figure 7.1: A Reacting Particle in the Multiple Surface Reactions Model. and
are the temperatures in Equation 12–159.
Based on the analysis above, Ansys Fluent uses the following equation to describe
the rate of reaction of a particle surface species
with the gas phase species
. The reaction stoichiometry of reaction
in this case is described by
(7–101) |
and the rate of reaction is given as
(7–102) |
(7–103) |
| where, | |
|
| |
|
| |
|
| |
|
| |
|
| |
|
| |
|
| |
|
| |
|
|
The effectiveness factor, , is related to the surface area, and can be used in each
reaction in the case of multiple reactions.
is given by
(7–104) |
The kinetic rate of reaction is defined as
(7–105) |
The rate of the particle surface species depletion for reaction order
is given by
(7–106) |
For reaction order ,
(7–107) |
When more than one gas phase reactant takes part in the reaction, the reaction stoichiometry must be extended to account for this case:
(7–108) |
To describe the rate of reaction of a particle surface species
in the presence of
gas phase species
, it is necessary to define the diffusion-limited species for
each solid particle reaction, that is, the species for which the concentration
gradient between the bulk and the particle surface is the largest. For the rest
of the species, the surface and the bulk concentrations are assumed to be equal.
The concentration of the diffusion-limited species is shown as
and
in Figure 7.1: A Reacting Particle in the Multiple Surface Reactions Model, and the
concentrations of all other species are denoted as
. For stoichiometries with multiple gas phase reactants, the
bulk partial pressure
in Equation 7–103 and Equation 7–106 is the bulk partial pressure of the
diffusion-limited species,
for reaction
.
The kinetic rate of reaction is then defined as
(7–109) |
| where, | |
|
| |
|
|
When this model is enabled, the constant (Equation 7–104) and the
effectiveness factor
(Equation 7–102) are entered in the
Reactions dialog box (see User Inputs for Particle Surface Reactions in the User's Guide).
Reactions involving only particle surface reactants can be modeled, provided that the particle surface reactants and products exist on the same particle.
The reaction rate for this case is given by Equation 7–107.
The decomposition reactions of particle surface species can be modeled.
(7–110) |
The reaction rate for this case is given by Equation 7–102 – Equation 7–109, where the diffusion-limited species is now the gaseous product of the reaction. If there are more than one gaseous product species in the reaction, it is necessary to define the diffusion-limited species for the particle reaction as the species for which the concentration gradient between the bulk and the particle surface is the largest.
The deposition reaction of a solid species on a particle can be modeled with the following assumptions:
(7–111) |
The theoretical analysis and Equation 7–102 – Equation 7–109 are applied for the surface reaction rate calculation, with the mass fraction of the surface species set to unity in Equation 7–102, Equation 7–106, and Equation 7–107.
In Ansys Fluent, for the particle surface species to be deposited on a particle, a finite mass of the species must already exist in the particle. This allows for activation of the deposition reaction selectively to particular injection particles. It follows that, to initiate the solid species deposition reaction on a particle, the particle must be defined in the Set Injection Properties Dialog Box (or Set Multiple Injection Properties Dialog Box) to contain a small mass fraction of the solid species to be deposited. For details on defining the particle surface species mass fractions, see Using the Multiple Surface Reactions Model for Discrete-Phase Particle Combustion in the User’s Guide.
Reactions of gaseous species catalyzed on the particle surface can also be modeled following Equation 7–102 – Equation 7–109 for the surface reaction rate calculation, with the mass fraction of the surface species set to unity in Equation 7–102, Equation 7–106, and Equation 7–107. To apply this type of reaction, see Modeling Gaseous Solid Catalyzed Reactions in the User's Guide. For details on defining the particle surface species mass fractions, see Using the Multiple Surface Reactions Model for Discrete-Phase Particle Combustion in the User’s Guide.
Components of multicomponent particles can participate in chemical reactions. Reactions can take place between particle components, or with a gas phase component, and particle components are allowed to evaporate and react in sequence or simultaneously. Several reaction models are available as built-in functionality as well as a user defined option. The multicomponent particle may contain reacting, volatile, or inert components that do not participate in any reactions.
Similarly to the combusting particle solid components, the multicomponent particle components can participate in chemical reactions as described below.
Particle species reacts with one or more gas-phase species
Particle species reacts with one or more particle species
Particle species decomposes to gas phase species
A gas phase species is converted to a particle species and is deposited on the particle
Catalytic reactions where the gas phase species react on the particle surface without consuming a particle species
For these reaction types, the following reaction models are used in Ansys Fluent:
kinetics/diffusion
The reaction rate is calculated according to Ansys Fluent Model Formulation.
surface-kinetics
The reaction rate is calculated as:
(7–112)
where:
= rate of reaction
of particle component
(kg/s)
= surface area of the particle (m2).
= effectiveness factor.
= reaction rate coefficient (kg/(m2s)).
= mass fraction of particle component
.
= partial pressure of gas reactant
.
= reaction order for gas reactant
.
The reaction rate coefficient
is expressed as:
(7–113)
where:
= pre-exponential factor. The factor must be specified in SI units consistent with those used for the reaction rate on a kg basis.
= temperature of the particle (K).
= exponent on temperature.
= activation energy (J/Kg-mol/K).
= ideal gas constant.
volume-kinetics
The reaction rate is calculated as:
(7–114)
where:
= rate of reaction
of particle component
(kg/s)
= particle volume (m3).
= molecular weight of particle component
(Kg/Kg-mol)
= reaction rate coefficient. The reaction rate coefficient has the same form as Equation 7–113, with the units of the pre-exponential factor
specified on a kg-mol basis.
and
= concentrations of gas reactant
and particle reactant
, respectively (Kg-mol/m3).
and
= reaction orders for gas reactant
and particle reactant
, respectively.
phase-change
In this reaction model, particle component can evaporate con-currently with the other reacting components. Only one solid/condensed particle material is allowed in the reaction stoichiometry as reactant and one gas material as product. The phase-change rate of particle component
to gas component
is calculated according to:
(7–115)
= rate of phase-change of particle component
(kg/s)
= surface area of the particle (m2)
= molecular weight of particle component
(Kg/Kg-mol)
= mass transfer coefficient (m/s)
and
= concentrations of particle reactant
at the particle surface and gas phase species
, respectively (Kg-mol/m3)
thermolysis (free stream particles only)
(7–116)
where
= rate of reaction
of particle component
(kg/s)
= pre-exponential factor (in consistent SI units on a kg basis).
= particle diameter (m).
= temperature of the particle (K).
= exponent on temperature.
= activation energy (J/Kg-mol/K).
= ideal gas constant.
In addition to the above reaction models, you can define your own reaction rate using a user-defined function. For more information on using these models, see Multicomponent Particles with Chemical Reactions in the Fluent User's Guide.