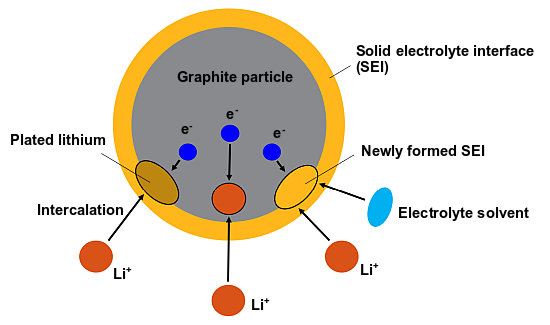
In Ansys Fluent, the physics-based battery life model considers the aging mechanisms of the growth of the solid electrolyte interphase (SEI) and the decomposition of metallic lithium (Li plating) [723]. Figure 19.5: Electrochemical Reactions on the Particle Surface schematically shows the electrochemical reactions that take place on the particle surface in the anode during battery charging.
The following electrochemical reactions are considered in the physics-based battery life model:
The reactions occur when the ions are deposited onto the graphite surface and are partly converted to
metallic lithium (
plating) and partly to SEI.
The SEI formation and lithium deposition reactions are unwanted side reactions that compete with the intercalation reaction. These side reactions are only prominent and modeled in the anode during battery charging.
The SEI growth and plating cause the battery to age mainly via the following effects:
Some of the lithium inventory is lost to form the SEI and plated lithium, which leads to a drop in the battery theoretical capacity.
The surface film of the SEI and plated lithium increases the surface resistance of the anode graphite particles.
The SEI and plated lithium reduce the porosity of the anode porous media, which further reduces the effective conductivity and diffusivity of the electrolyte.
To model the SEI growth and lithium plating, the physics-based battery life model solves
the evolution of the SEI concentration using the following formulation:
(19–54) |
and the plated lithium concentration using the following formulation:
(19–55) |
In Equation 19–54 and Equation 19–55, the following notations are used:
|
|
|
|
|
|
|
|
|
|
As the governing equations of SEI and plated lithium have no spatial term, their evolutions are solely dependent on the local source terms, that is, the local current fluxes of the side reactions. Those source terms are formulated by the cathodic Tafel expressions:
(19–56) |
and
(19–57) |
In the above equations:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The surface concentration of EC, , is determined by the diffusive flux of EC across the surface film:
(19–58) |
where
|
|
|
|
|
|
Similar to Equation 19–19, the overpotential of a
side reaction (SEI formation and lithium deposition) is computed as:
(19–59) |
where
|
|
|
|
|
|
|
|
The physical properties of the surface film (thickness and electric resistance
) are expressed as functions of the SEI and plated lithium
concentrations:
(19–60) |
(19–61) |
The anode electrolyte porosity decreases as the surface film grows and is computed as:
(19–62) |
In Equation 19–60 through Equation 19–62, the following notations are used:
|
|
|
|
|
|
|
|
|
|
|
|
In practical Newman’s P2D model simulations of battery aging through electric load cycles, the physics-based battery life model is solved on the electrode scale in the standalone mode (see Simulating the Aging Process of a Battery (Standalone Mode) in the Fluent User's Guide for more information). Further, the standalone simulation results can be included in a 3D CFD simulation to account for the battery aging effects. In this scenario, the aging effects are treated as being frozen in time. More specifically, during the E-chemistry model calculations, the distributions of the SEI and the plated lithium concentrations (which determines the lithium inventory loss), the surface film resistance, and the electrolyte porosity across the anode are included into the Newman’s P2D model as steady-state profiles. In a practical CFD simulation constrained by the computing power, the simulation time is much smaller than the time scale of battery life. This wide separation of time scale justifies the frozen-in-time aging effects. See Inputs for the Newman’s P2D Model in the Fluent User's Guide for details.