For information about restrictions and special cases for using the non-premixed model, see the following sections:
The unique dependence of (species mass fractions,
density, or temperature) on
(Equation 8–12 or Equation 8–14) requires that the reacting
system meet the following conditions:
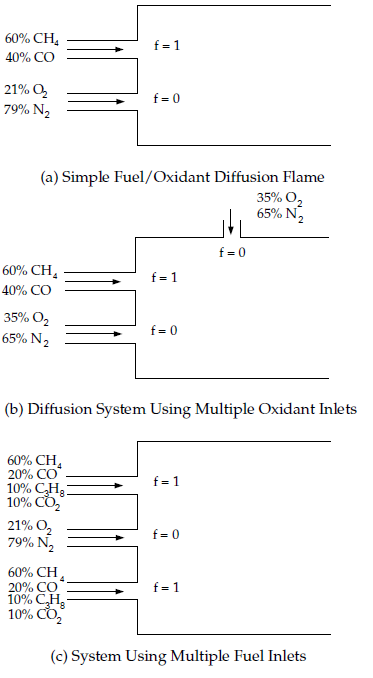
The chemical system must be of the diffusion type with discrete fuel and oxidizer inlets (spray combustion and pulverized fuel flames may also fall into this category).
The Lewis number must be unity. (This implies that the diffusion coefficients for all species and enthalpy are equal, a good approximation in turbulent flow).
When a single mixture fraction is used, the following conditions must be met:
Only one type of fuel is involved. The fuel may be made up of a burnt mixture of reacting species (for example, 90% CH4 and 10% CO) and you may include multiple fuel inlets. The multiple fuel inlets must have the same composition; two or more fuel inlets with different fuel composition are not allowed (for example, one inlet of CH4 and one inlet of CO). Similarly, in spray combustion systems or in systems involving reacting particles, only one off-gas is permitted.
Only one type of oxidizer is involved. The oxidizer may consist of a mixture of species (for example, 21% O2 and 79% N2) and you may have multiple oxidizer inlets. The multiple oxidizer inlets must, however, have the same composition. Two or more oxidizer inlets with different compositions are not allowed (for example, one inlet of air and a second inlet of pure oxygen).
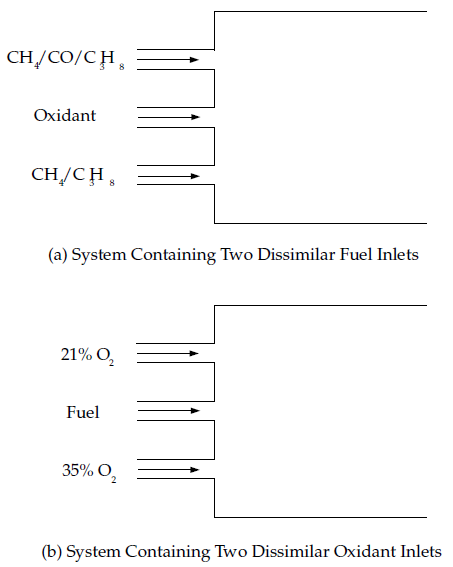
When two mixture fractions are used, three streams can be involved in the system. Valid systems are as follows:
Two fuel streams with different compositions and one oxidizer stream. Each fuel stream may be made up of a mixture of reacting species (for example, 90% CH4 and 10% CO). You may include multiple inlets of each fuel stream, but each fuel inlet must have one of the two defined compositions (for example, one inlet of CH4 and one inlet of CO).
Mixed fuel systems including gas-liquid, gas-coal, or liquid-coal fuel mixtures with a single oxidizer. In systems with a gas-coal or liquid-coal fuel mixture, the coal volatiles and char can be treated as a single composite fuel stream and the secondary stream can represent another fuel. Alternatively, for coal combustion, the volatile and char off-gases are tracked separately as distinct fuel streams.
Two oxidizer streams with different compositions and one fuel stream. Each oxidizer stream may consist of a mixture of species (for example 21% O2 and 79% N2). You may have multiple inlets of each oxidizer stream, but each oxidizer inlet must have one of the two defined compositions (for example, one inlet of air and a second inlet of pure oxygen).
A fuel stream, an oxidizer stream, and a non-reacting secondary stream.
The flow must be turbulent.
It is important to emphasize that these restrictions eliminate the use of the non-premixed approach for directly modeling premixed combustion. This is because the unburned premixed stream is far from chemical equilibrium. Note, however, that an extended mixture fraction formulation, the partially premixed model (see Partially Premixed Combustion), can be applied to non-premixed (with mixed-but-unburnt regions), as well as partially premixed flames.
Figure 8.12: Chemical Systems That Can Be Modeled Using a Single Mixture Fraction and Figure 8.13: Chemical System Configurations That Can Be Modeled Using Two Mixture Fractions illustrate typical reacting system configurations that can be handled by the non-premixed model in Ansys Fluent. Figure 8.14: Premixed Systems That Cannot Be Modeled Using the Non-Premixed Model shows a premixed configuration that cannot be modeled using the non-premixed model.
You can use the non-premixed model if your Ansys Fluent simulation includes liquid droplets and/or coal particles. In this case, fuel enters the gas phase within the computational domain at a rate determined by the evaporation, devolatilization, and char combustion laws governing the dispersed phase. In the case of coal, the volatiles and the products of char can be defined as two different types of fuel (using two mixture fractions) or as a single composite off-gas (using one mixture fraction), as described in Modeling Coal Combustion Using the Non-Premixed Model in the User’s Guide.
While most problems you solve using the non-premixed model will
involve inlets that contain either pure oxidant or pure fuel ( or 1), you can
include an inlet that has an intermediate value of mixture fraction
(
) provided that this inlet represents a completely
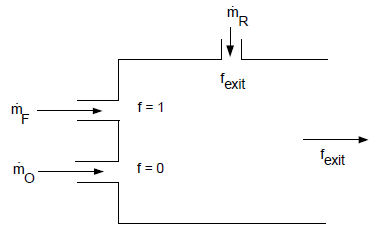
reacted mixture. Such cases arise when there is flue gas recirculation,
as depicted schematically in Figure 8.15: Using the Non-Premixed Model with Flue Gas Recycle. Since
is a conserved quantity, the mixture fraction at
the flue gas recycle inlet can be computed as
(8–31) |
or
(8–32) |
where is the exit mixture fraction (and the mixture fraction
at the flue gas recycle inlet),
is
the mass flow rate of the oxidizer inlet,
is the mass flow rate of the
fuel inlet,
is the mass flow rate of the
recycle inlet.
If a secondary stream is included,
(8–33) |
and
(8–34) |
To model the effect of dilution on combustion without the expense of using two mixture fractions, Ansys Fluent allows the introduction of an inert stream into the domain. Unlike a secondary mixture fraction, the inert does not chemically equilibrate with the primary fuel and oxidizer - instead, its composition remains constant after mixing. However the inert stream does affect the solution due to its influence on enthalpy, specific heat, and density of the mixture. The equation for conservation of inert is written as:
(8–35) |
|
where | |
|
| |
|
| |
|
| |
|
|
Equation 8–35 has no sources or sinks, because the problem is reduced to tracking a conserved scalar when it is assumed that the inert components have the same turbulent diffusivities.
The mixture properties are computed from the mean () and variance (
) of the mixture fraction in the cell, the reaction
progress variable (
, when the partially premixed model is enabled),
the cell enthalpy (
, for non-adiabatic flows), and the inert tracer
(
). The mixture is modeled as
a blend of inert and active species, but the PDF tables need to be
accessed with conditioned variables. Conditioning
is necessary to take into account the volume taken up by the inert
fraction, yet still be able to use previously built tables by straightforward
lookup. The mean mixture fraction and mixture fraction variance used
to access the PDF table is given by:
(8–36) |
(8–37) |
The reaction progress variable is not conditioned, however the
cell enthalpy must be conditioned to account for the inert enthalpy.
The inert enthalpy and active enthalpy are obtained from the following
relationships:
(8–38) |
where is the enthalpy of the cell at temperature
,
is the enthalpy of the active mixture-fraction stream
and
is the enthalpy of the inert
stream. Here it is assumed that the inert and the active streams have
the same temperature, but different enthalpies. To calculate the temperature
in the cell, Equation 8–38 is solved
for the temperature and for
,
which gives the partitioning of the energy between the inert and active
streams.
The inert enthalpy is defined as
(8–39) |
where refers to the mass fraction of species
defined in the inert stream,
is the formation enthalpy of species
,
is the reference temperature,
the specific heat of species
, and
is the number of inert species.
The inert and PDF enthalpies are defined further in Equation 42–11 in the User's Guide.
The specific heat of the mixture is evaluated by mixing the inert and active streams in the following way:
(8–40) |
The density of the mixture is calculated by using a harmonic average of the densities of the active and inert streams, weighted by the inert tracer:
(8–41) |
Here, the inert density () is calculated from
the ideal gas law.
For information on how to set up the inert model, see Setting Up the Inert Model in the User's Guide.