As mentioned in Thermal Abuse Model, thermal runaway reactions could occur when a battery is exposed to abnormal conditions such as overcharging, internal short circuits, or overheating. As the battery experiences thermal runaway reactions, in addition to excessive heat release, some flammable gases would be released.
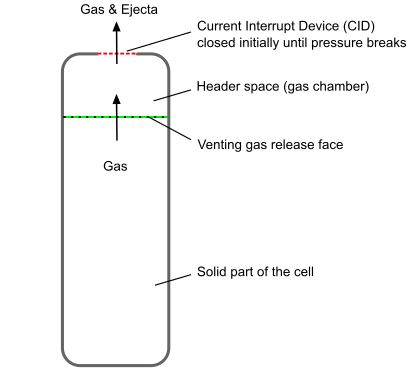
Modern batteries, especially lithium-ion batteries, often contain safety mechanisms such as pressure relief vents or a current interrupt device (CID) as schematically shown in Figure 19.4: Battery with Safety Mechanism.
During thermal runaway reactions, venting gases are generated as products of decomposition reactions. The gases seep through the battery active zone and build up in the battery header space. When the pressure inside the header reaches a critical value, these safety features activate. The venting mechanisms allow excess gas to escape, preventing a catastrophic failure.
The venting process can be simulated in Ansys Fluent. The release rate of venting gases is assumed to be directly proportional to the rate of thermal abuse reactions.
In the one-equation abuse model, the total venting gas release rate from the battery is computed as:
(19–42)
where
= total gas release from the battery if the abuse reaction is complete
= active zone volume
= possible range of abuse reaction
= abuse reaction rate in Equation 19–33
In the four-equation abuse model, the venting gas release rate from the battery is computed as:
(19–43)
where
,
,
, and
are the total venting gas released (in kg) from each of the four abuse reactions, respectively;
,
,
, and
are the ranges of four reaction progress variables;
,
,
, and
are the abuse reaction rates in Equation 19–39.
Venting gas is produced inside the battery active zone. The gas seeps through and accumulates in the header space of the battery. In Ansys Fluent, the battery active zone is modeled as solid. The venting gas is assumed to be released from the interface where the venting gas cavity contacts the battery active zone.
The venting gas temperature is calculated as the weighted average heat-release temperature:
(19–44) |
where is the heat generation rate of the thermal abuse reactions computed from Equation 19–34 for the one-equation abuse model and from Equation 19–39 for the four-equation model.
Besides relating the venting release rate to the abuse reaction rate, Ansys Fluent also allows you to define venting gas release rate and the release temperature from a file directly, which can be derived from experimental data.
When the venting model is used, the species transport model is enabled automatically. The venting gases are produced during thermal runaway reactions. The venting gas mixture usually contains carbon dioxide, carbon monoxide, hydrogen, and some other hydrocarbons. Depending on the battery type, the venting gas composition may vary. By default, a mixture material called venting-gas-mixture is used with its composition taken from [302] (see Table 19.1: Species Composition of The Venting-Gas-Mixture).
Table 19.1: Species Composition of The Venting-Gas-Mixture
| Species Composition | Mass Fraction |
|---|---|
| CO | 0.536 |
| CO2 | 0.335 |
| H2 | 0.023 |
| CH4 | 0.062 |
| C2H4 | 0.033 |
| C2H6 | 0.011 |
| H2O | 0 |
| O2 | 0 |
| N2 | 0 |
Some species in the venting gas mixture are combustible. Chemical reactions can occur if the temperature is high enough. As in [302], the following five reactions are pre-defined for the venting gas mixture:
The reaction model can be used along with the battery venting model.
For more information about using the battery venting model, see, Battery Venting Model in the Fluent User's Guide.