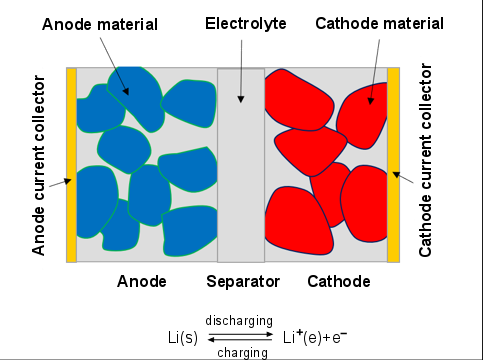
Lithium-ion battery is typically made by stacking/rolling-up many layers of the electrodes to form a sandwich-like structure. Each electrode consists of the anode current collector, anode, separator, cathode, and cathode current collector as shown in Figure 18.1: Schematic structure of an electrode pair.
During discharge, lithium stored in the anode material diffuses out, undergoes electrochemical reaction at the solid-electrolyte interface (SEI), and dissolves in the electrolyte phase as lithium-ion species. Then lithium-ion species diffuses further from the anode side to the cathode side. As the lithium-ion species undergoes electrochemical reaction at the cathode SEI, lithium is intercalated into the cathode material. During the charge process, the lithium and lithium-ion move in the opposite direction.
The detailed lithium-ion battery model simulates the detailed physics occurring in the charging and discharging processes from first principles. The model is derived from the concentrated solution theory [474], [212]. The governing equations are the mass and charge balance equations expressed as:
where
|
|
|
|
|
|
The mass and electric current flux can be computed as:
where
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
is defined by:
(18–8) |
where
|
|
|
|
|
|
The electrochemical reaction occurs at the SEIs. The reaction rate is determined by the Butler-Volmer equation:
(18–9) |
A linearized form can also be used:
(18–10) |
where
|
|
|
|
|
|
|
|
At the SEIs, although potential and lithium are not continuous, the mass and electric current fluxes must be continuous satisfying the following conditions:
(18–11) |
(18–12) |
Substituting the flux equations (Equation 18–6 and Equation 18–7) into the mass and current conservation equations (Equation 18–4 and Equation 18–4) yields the following:
Mass conservation law:
Charge conservation law:
Ansys Fluent solves Equation 18–13 through Equation 18–16 together with the SEI conditions (Equation 18–11 and Equation 18–12 to obtain the electric and species fields.
For information about using the lithium-ion battery model, see Simulating the Lithium-ion Battery in the Fluent User's Guide.