The selective noncatalytic reduction of NOx (SNCR), first described by Lyon [403], is a method to reduce the emission of NOx from combustion by injecting a selective reductant such as ammonia (NH3) or urea (CO(NH2)2) into the furnace, where it can react with NO in the flue gas to form N2. However, the reductant can be oxidized as well to form NOx. The selectivity for the reductive reactions decreases with increasing temperature [439], while the rate of the initiation reaction simultaneously increases. This limits the SNCR process to a narrow temperature interval, or window, where the lower temperature limit for the interval is determined by the residence time.
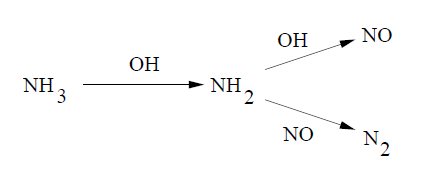
Several investigators have modeled the process using a large number of elementary reactions. A simple empirical model has been proposed by Fenimore [172], which is based on experimental measurements. However, the model was found to be unsuitable for practical applications. Ostberg and Dam-Johansen [501] proposed a two-step scheme describing the SNCR process as shown in Figure 9.2: Simplified Reaction Mechanism for the SNCR Process, which is a single initiation step followed by two parallel reaction pathways: one leading to NO reduction, and the other to NO formation.
(9–88) |
(9–89) |
The reaction orders of NO and NH3 at 4% volume
O2 and the empirical rate constants and
for Equation 9–88
Equation 9–89, respectively, have been estimated from work done by
Brouwer et al. [82]. The reaction order of NO was found to be 1 for
Equation 9–88 and the order of NH3 was also
found to be 1 for both reactions. As such, the following reaction rates for NO and
NH3, at 4% volume O2, were proposed:
(9–90) |
(9–91) |
The rate constants and
have
units of m3/mol-s, and are defined as
where J/mol and
J/mol.
This model has been shown to give reasonable predictions of the SNCR process in pulverized coal and fluidized bed combustion applications. The model also captures the influence of the most significant parameters for SNCR, which are the temperature of the flue gas at the injection position, the residence time in the relevant temperature interval, the NH3 to NO molar ratio, and the effect of combustible additives. This model overestimates the NO reduction for temperatures above the optimum temperature by an amount similar to that of the detailed kinetic model of Miller and Bowman [439].
Important: The SNCR process naturally occurs when NH3 is present in the flame as a fuel N intermediate. For this reason, even if the SNCR model is not activated and there is no reagent injection, the natural SNCR process may still occur in the flame. The temperature range or “window” at which SNCR may occur is 1073 K < T < 1373 K. If you want to model your case without using the natural SNCR process, contact your support engineer for information on how to deactivate it.
Urea as a reagent for the SNCR process is similar to that of injecting ammonia, and has been used in power station combustors to reduce NO emissions successfully. However, both reagents, ammonia and urea, have major limitations as a NOx reducing agent. The narrow temperature “window” of effectiveness and mixing limitations are difficult factors to handle in a large combustor. The use of urea instead of ammonia as the reducing agent is attractive because of the ease of storage and handling of the reagent.
The SNCR process using urea is a combination of Thermal DeNOx (SNCR with ammonia) and RAPRENOx (SNCR using cyanuric acid that, under heating, sublimes and decomposes into isocyanic acid), because urea most probably decomposes into ammonia and isocyanic acid [439].
One problem of SNCR processes using urea is that slow decay of HNCO, as well as the reaction channels leading to N2O and CO, can significantly increase the emission of pollutants other than NO. Urea seems to involve a significant emission of carbon-containing pollutants, such as CO and HNCO.
Also, some experimental observations [560] show that SNCR using urea is
effective in a narrow temperature window that is shifted toward higher temperatures, when
compared to Thermal DeNOx processes at the same value of the ratio of nitrogen in the reducing
agent and the NO in the feed, , where
is defined as the ratio of nitrogen in the reducing agent and NO in the feed.
The effect of increasing the
value is to increase the efficiency of abatement, while the effect of
increasing O2 concentration depends on the temperature.
The model described here is proposed by Brouwer et al. [82] and is a seven-step reduced kinetic mechanism. Brouwer et al. [82] assumes that the breakdown of urea is instantaneous and 1 mole of urea is assumed to produce 1.1 moles of NH3 and 0.9 moles of HNCO. The work of Rota et al. [560] proposed a finite rate two-step mechanism for the breakdown of urea into ammonia and HNCO.
The seven-step reduced mechanism is given in Table 9.2: Seven-Step Reduced Mechanism for SNCR with Urea, and the two-step urea breakdown mechanism is given in Table 9.3: Two-Step Urea Breakdown Process.
Table 9.2: Seven-Step Reduced Mechanism for SNCR with Urea
| Reaction | A | b | E |
|---|---|---|---|
|
| 4.24E+02 | 5.30 | 349937.06 |
|
| 3.500E-01 | 7.65 | 524487.005 |
|
| 2.400E+08 | 0.85 | 284637.8 |
|
| 1.000E+07 | 0.00 | -1632.4815 |
|
| 1.000E+07 | 0.00 | 0 |
|
| 2.000E+06 | 0.00 | 41858.5 |
|
| 6.900E+17 | -2.5 | 271075.646 |
where SI units (m, mol, sec, J) are used in Table 9.2: Seven-Step Reduced Mechanism for SNCR with Urea and Table 9.3: Two-Step Urea Breakdown Process.
When the SNCR model with urea injection is employed in addition to the usual transport equations, Ansys Fluent solves the following three additional mass transport equations for the urea, HNCO, and NCO species.
(9–92) |
(9–93) |
(9–94) |
where ,
and
are mass fractions of urea, HNCO, and NCO in the gas phase. The source terms
,
, and
are determined according to the rate equations given in Table 9.2: Seven-Step Reduced Mechanism for SNCR with Urea and Table 9.3: Two-Step Urea Breakdown Process and the
additional source terms due to reagent injection. These additional source terms are determined
next. The source terms in the transport equations can be written as follows:
(9–95) |
(9–96) |
(9–97) |
Apart from the source terms for the above three species, additional source terms for NO, NH3, and N2O are also determined as follows, which should be added to the previously calculated sources due to fuel NOx:
(9–98) |
(9–99) |
(9–100) |
The source terms for
species
are determined from the rate equations given in Table 9.2: Seven-Step Reduced Mechanism for SNCR with Urea and Table 9.3: Two-Step Urea Breakdown Process.
The rate of urea production is equivalent to the rate of reagent release into the gas phase through droplet evaporation:
(9–101) |
where is the rate
of reagent release from the liquid droplets to the gas phase (kg/s)
and
is the cell volume (m3).
If the urea decomposition model is set to the user-specified option, then the rate of NH3 production is proportional to the rate of reagent release into the gas phase through droplet evaporation:
(9–102) |
where is the rate of reagent release from the liquid droplets to the gas phase
(kg/s),
is the mole fraction of NH3 in the
NH3/ HNCO mixture created from urea decomposition, and
is the cell volume (m3).
If the urea decomposition model is set to the user-specified option, then the rate of HNCO production is proportional to the rate of reagent release into the gas phase through droplet evaporation:
(9–103) |
where , the injection source term, is the rate of reagent release from the liquid
droplets to the gas phase (kg/s),
is the mole fraction of HNCO in the NH3/ HNCO mixture
created from urea decomposition, and
is the cell volume (m3).
Important: The mole conversion fractions (MCF) for species NH3 and HNCO are
determined through the user species values such that if one mole of urea decomposes into 1.1
moles of NH3 and 0.9 moles of HNCO, then = 0.55 and
= 0.45. When the user-specified option is used for urea
decomposition, then
.
However, the default option for urea decomposition is through
rate limiting reactions given in Table 9.3: Two-Step Urea Breakdown Process, and the source terms are calculated
accordingly. In this case, both values of and
are zero.