The design of complex combustion systems for utility boilers, based on air- and fuel-staging technologies, involves many parameters and their mutual interdependence. These parameters include the local stoichiometry, temperature and chemical concentration field, residence time distribution, velocity field, and mixing pattern. A successful application of the in-furnace reduction techniques requires control of these parameters in an optimum manner, so as to avoid impairing the boiler performance. In the mid 1990s, global models describing the kinetics of NOx destruction in the reburn zone of a staged combustion system became available. Two of these models are described below.
The instantaneous NOx reburning mechanism is a pathway whereby NO reacts with hydrocarbons and is subsequently reduced. In general:
(9–70) |
Three reburn reactions are modeled by Ansys Fluent for :
(9–71) |
(9–72) |
(9–73) |
Important: If the temperature is outside of this range, NO reburn will not be computed.
The rate constants for these reactions are taken from Bowman [72] and have units of m3/mol-s:
The NO depletion rate due to reburn is expressed as
(9–74) |
and the source term for the reburning mechanism in the NO transport equation can be calculated as
(9–75) |
Important: To calculate the NO depletion rate due to reburning, Ansys Fluent will obtain the concentrations of CH, CH2, and CH3 from the species mass fraction results of the combustion calculation. When you use this method, you must be sure to include the species CH, CH2, and CH3 in your problem definition.
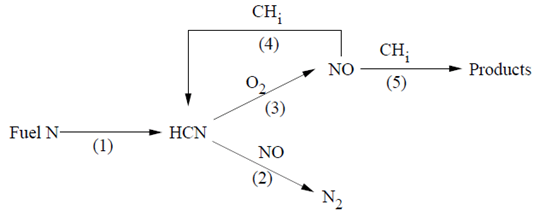
The partial equilibrium approach is based on the model proposed by Kandamby et al. [289], [21]. The model adds a reduction path to De Soete’s global model [139] that describes the NOx formation/destruction mechanism in a pulverized coal flame. The additional reduction path accounts for the NOx destruction in the fuel-rich reburn zone by CH radicals (see Figure 9.1: De Soete’s Global NOx Mechanism with Additional Reduction Path).
This model can be used in conjunction with the eddy-dissipation combustion model and does not require the specification of CH radical concentrations, because they are computed based on the CH-radical partial equilibrium. The reburn fuel itself can be an equivalent of CH4, CH3, CH2, or CH. How this equivalent fuel is determined is open for debate, and an approximate guide would be to consider the C/H ratio of the fuel itself. A multiplicative constant of 4.0x10-4 has been developed for the partial equilibrium of CH radicals to reduce the rates of HCN and NO in the reburn model. This value was obtained by researchers who developed the model, by way of predicting NOx values for a number of test cases for which experimental data exists.
In the fuel-rich reburn zone, the HCN oxidation is suppressed and the amount of NO formed in the primary combustion zone is decreased by the reduction reaction from HCN to N2. However, the NO concentration may also decrease due to reactions with CH radicals, which are available in significant amounts in the reburn zone. The following are considered to be the most important reactions of NO reduction by CH radicals:
(9–76) |
(9–77) |
(9–78) |
These reactions may be globally described by the addition of pathways (4) and (5) in Figure 9.1: De Soete’s Global NOx Mechanism with Additional Reduction Path, leading respectively to the formation of HCN and of minor intermediate nitrogen radicals. Assuming that methane is the reburning gas, the global NO reduction rates are then expressed as
(9–79) |
(9–80) |
where
Therefore, the additional source terms of the HCN and NO transport equations due to reburn reactions are given by
(9–81) |
(9–82) |
Certain assumptions are required to evaluate the rate constants ,
, and
and the factors
and
. For hydrocarbon
diffusion flames, the following reaction set can be reasonably considered
to be in partial equilibrium:
(9–83) |
(9–84) |
(9–85) |
(9–86) |
Thus, the rate constants may be computed as
where ,
, and
are the rate constants for Equation 9–76 –
Equation 9–78. The forward and reverse rate constants for Equation 9–83 – Equation 9–86 are
–
and
–
, respectively. In addition, it is assumed that
, because the H-radical concentration in the post-flame region of a
hydrocarbon diffusion flame has been observed to be of the same order as
[H2]. Finally, the OH-radical concentration is estimated by considering
the reaction
(9–87) |
to be partially equilibrated, leading to the relationship
Values for the rate constants ,
, and
for different equivalent fuel types are given in Arrhenius form
(
) in Table 9.1: Rate Constants for Different Reburn Fuels
[357]. All rate constants have units of
m3/mol-s, and all values of
have units of J/mol.
Table 9.1: Rate Constants for Different Reburn Fuels
| Equivalent Fuel Type |
|
|
| ||||||
|
|
|
|
|
|
|
|
|
| |
| CH4 |
| -1.54 | 27977 |
| -3.33 | 15090 |
| -2.64 | 77077 |
| CH3 |
| -1.54 | 27977 |
| -3.33 | 15090 |
| -2.64 | 77077 |
| CH2 |
| -1.54 | 27977 |
| -3.33 | 15090 |
| -2.64 | 77077 |
| CH | 0.0 | 0.0 | 0.0 |
| -3.33 | 15090 |
| -2.64 | 77077 |
For Equation 9–87,