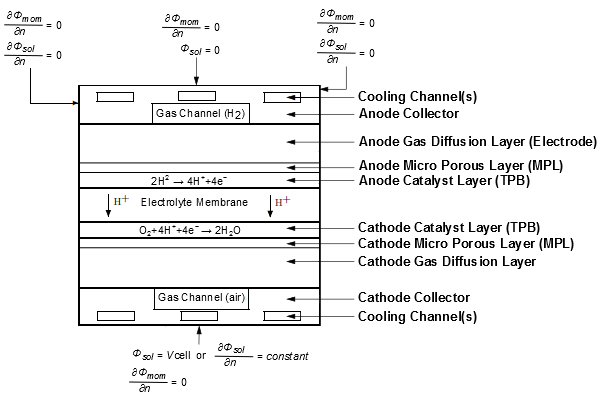
At the center of the electrochemistry is the computation of the rates of the anodic and cathodic reactions. The electrochemistry model adopted in Ansys Fluent is one that has been used by other groups ([325], [419], and [663]).
The driving force behind these reactions is the surface overpotential: the difference
between the phase potential of the solid and the phase potential of the electrolyte/membrane.
Therefore, two potential equations are solved. One potential equation (Equation 20–3) accounts for the electron transport of through the solid conductive materials and is solved in the TPB catalyst
layer, the solid grids of the porous media, and the current collector; the other potential
equation (Equation 20–4) represents the protonic (that is, ionic)
transport of
and is solved in the TPB catalyst layer and the membrane. The two potential
equations are as follows:
(20–3) |
(20–4) |
|
where | |
|
| |
|
| |
|
|
In the above equations, subscripts and
refer to the membrane and solid phases, respectively.
The following figure illustrates the boundary conditions that are used to solve for
and
.
There are two types of external boundaries: those that have an electrical current passing through them, and those that do not.
As no ionic current leaves the fuel cell through any external boundary, there is a zero
flux boundary condition for the membrane phase potential, , on all outside boundaries.
For the solid phase potential, , there are external boundaries on the anode and the cathode side that are in
contact with the external electric circuit. Electrical current generated in the fuel cell only
passes through these boundaries. On all other external boundaries there is a zero flux boundary
condition for
.
On the external contact boundaries, fixed values for (potentiostatic boundary conditions) are recommend. If the anode side is set
to zero, the (positive) value prescribed on the cathode side is the cell voltage. Specifying a
constant flux (say on the cathode side) means to specify galvanostatic boundary
conditions.
The transfer currents, or the source terms in Equation 20–3 and Equation 20–4, are nonzero only inside the catalyst layers and are computed as:
For the potential equation in the solid phase,
on the anode side and
on the cathode side.
For the potential equation in the membrane phase,
on the anode side and
on the cathode side.
The source terms in Equation 20–3 and Equation 20–4, also called the exchange current density (A/m3), have the following general definitions:
(20–5) |
(20–6) |
|
where | |
|
| |
|
| |
|
| |
|
| |
|
| |
|
| |
|
| |
|
| |
|
| |
|
| |
|
|
The above equation is the general formulation of the Butler-Volmer function. Note that the effects of the number of electrons in electrochemistry reactions are accounted for in the transfer coefficients. A simplification to this is the Tafel formulation given by:
(20–7) |
(20–8) |
By default, the Butler-Volmer function is used in the Ansys Fluent PEMFC model to compute the
transfer currents inside the catalyst layers. When the magnitude of the surface over-potential
() is large, the Butler-Volmer formulation reduces to the Tafel
formulation.
In Equation 20–5 through Equation 20–8, and
represent the molar concentration of the species upon which the anode and
cathode reaction rates depend, respectively. That is,
represents
and
represents
.
The reference exchange current density and
are dependent on the local temperature as follows:
(20–9) |
(20–10) |
|
where | |
|
| |
|
| |
|
|
The driving force for the kinetics is the local surface overpotential, , also known as the activation loss. It is generally the
difference between the solid and membrane potentials,
and
.
(20–11) |
(20–12) |
The half cell potentials at anode and cathode and
are computed by Nernst equations as follows [586]:
(20–13) |
(20–14) |
where is the water saturation pressure (Equation 20–54) and
,
, and
are the partial pressures of hydrogen, oxygen, and water vapor, respectively.
In the above equations, the standard state (
,
), the reversible potentials
and
, and the reaction entropies
and
are user-specified quantities.
From Equation 20–3 through Equation 20–14, the two potential fields can be obtained.
When Equation 20–8 is used to compute the cathode transfer current, the mass transport resistance in the catalyst microstructure is not considered ([586]). The resistance may consist of two parts:
Resistance due to an ionomer film
Resistance due to a liquid water film surrounding particles
In the Ansys Fluent PEMFC model, including these resistances in calculations of the transfer current is optional. The volumetric transfer current inside the cathode layers is represented by:
(20–15) |
where is the concentration of oxygen at the wall. The
is a user-specified value, and the
is calculated by:
(20–16) |
|
where | |
|
| |
|
| |
|
| |
|
| |
|
|
The in Equation 20–15 is calculated as:
(20–17) |
Here, is the ideal transfer current computed using Equation 20–6, but without considering resistance.