The Solid Oxide Fuel Cell (SOFC) With Unresolved Electrolyte Model is provided as an add-on module with the standard Ansys Fluent licensed software.
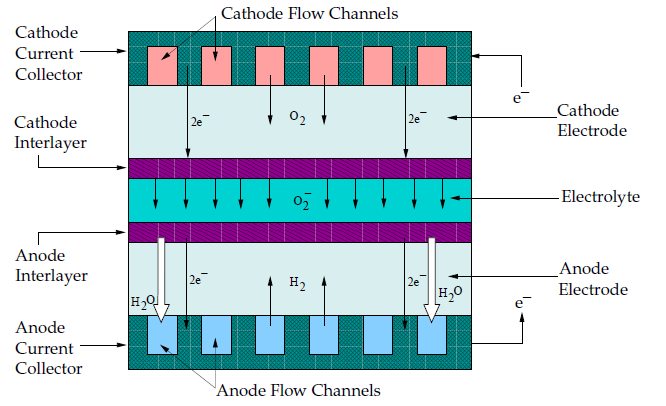
A fuel cell is an energy conversion device that converts the chemical energy of fuel into the electrical energy. A schematic of a solid oxide fuel cell (SOFC) is shown in Figure 20.5: Schematic of a Solid Oxide Fuel Cell.
As noted in [2], a solid oxide fuel cell is typically composed of an anode, cathode, and an electrolyte. Multiple fuel cells can be connected together, or stacked, using electrical interconnects. The electrolyte material must be solid, that is, non-porous, and exhibit a high ionic conductivity.
Note that the reason this modeling approach is referred to as the “SOFC Model with Unresolved Electrolyte" model is that the anode and the cathode “interlayers" and “electrolyte" (as shown in Figure 20.5: Schematic of a Solid Oxide Fuel Cell) are not actually included in the computational domain. They are modeled as a pair of wall and wall-shadow faces, named “electrolyte interfaces," with the species and energy sources and sinks due to the electrochemical reactions added to the adjacent computational cells.
All components of the fuel cell must have similar thermal expansion in order to minimize thermal stresses, which may cause cracking and de-lamination during thermal cycling. In addition, the components must be chemically stable in order to limit chemical interactions with other cell components.
A solid oxide fuel cell works by having electrically conducting porous ceramic electrodes attached on each side of an ionically conducting ceramic material. At the cathode/electrolyte/gas interface, also known as the triple phase boundary, oxygen is reduced to oxygen ions. The oxygen ions are conducted through the oxygen vacancies in the electrolyte to the anode side. At the anode/electrolyte/gas interface, oxygen ions combine to react with hydrogen at the anode electrode to form water and release electrons. The electrons travel through an external circuit to a load and back to the cathode electrode to close the circuit.
The Ansys Fluent SOFC With Unresolved Electrolyte Model provides the following features:
Local electrochemical reactions coupling the electric field and the mass, species, and energy transport.
Electric field solution in all porous and solid cell components, including ohmic heating in the bulk material.
Ability to handle
and combined
electrochemistry.
Inclusion of tortuosity for porous regions
Treatment of an arbitrary number of electrochemical cells arranged as a stack.
Significant geometric flexibility for treating planar, tubular, and other nonstandard SOFC configuration.
Use of non-conformal interface meshing (as a long as these interfaces are not the electrolyte interfaces).