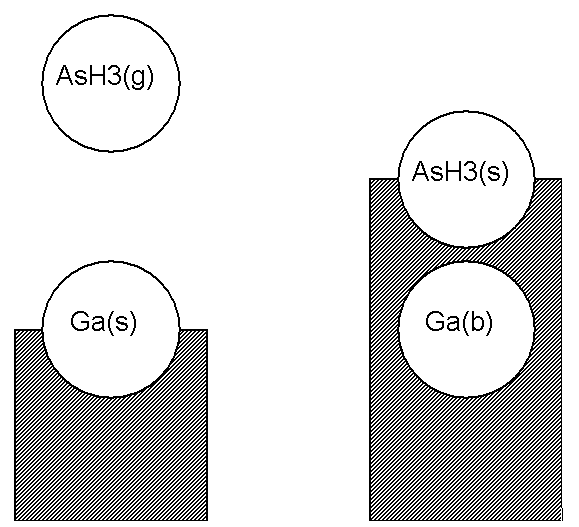
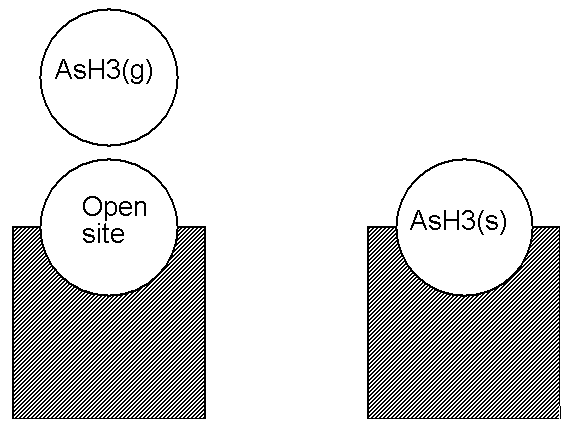
In this section, we consider in more detail how to write chemical reactions involving surface and bulk species. For clarity, we will write our rate expressions using a suffix (g) on our gas-phase species symbolic names, (s) for surface site species, and (b) for bulk-phase species.
A chemical species on the top layer of the solid, that is, a surface species, occupies a site. For example, an arsine molecule adsorbed on a surface could occupy a site, and might be denoted AsH3 (s). Another example might be a bare gallium atom, Ga(s), on top of a gallium arsenide (bulk) crystal. If another species, say a gas-phase AsH3 (g), lands on top of the Ga(s), it might "stick" or adsorb on that site, as shown in Figure 4.1: Illustration of an Adsorption Reaction using the Atomic Site Formalism . In this case the gallium atom that was at the surface is now covered up, such that it is no longer accessible to react with the gas and therefore no longer a surface species. In our nomenclature it has become a bulk species. The adsorbed AsH3 molecule now occupies the top-most layer at this site, so it has become the surface species AsH3 (s). In our formalism, we might write the adsorption reaction in Figure 4.1: Illustration of an Adsorption Reaction using the Atomic Site Formalism as
(4–1) |
In this reaction, the number of sites included on the left-hand side of the reaction equals the number on the right-hand side; the reaction conserves sites.
Suppose that we had wanted to describe the reverse reaction, that is, desorption of AsH3 from the surface. We would then write the reaction as
(4–2) |
Here, Ga(b) is included as a reactant in order to achieve site and elemental balance. We refer to the formalism described in Equation 4–1 and Equation 4–2 as the Atomic Site Formalism.
An alternate way of posing the example reaction above is to consider the situation on the left side of Figure 4.1: Illustration of an Adsorption Reaction using the Atomic Site Formalism not as having a surface gallium atom on a site, but to say that this is really an "open" site at which some event may take place (see Figure 4.2: Illustration of an Adsorption Reaction using the Open Site Formalism ). From this viewpoint, we could write the reaction of Figure 4.2: Illustration of an Adsorption Reaction using the Open Site Formalism as
(4–3) |
where the symbol O(s) is used to denote an open site. In specifying thermodynamic data for O(s), we would include no elements in the composition. Since O(s) contains no elements, this reaction conserves both sites and elements. We refer to this alternative formalism described in Equation 4–3 as the Open Site Formalism.
The Atomic Site and Open Site Formalisms are equally valid ways of stating these surface reactions. Either is allowed by the Surface Kinetics Pre-processor. Personal preference or, perhaps, the nature of a particular problem might dictate one over the other. Note that an "open" site must be considered as a species and therefore must have thermodynamic data specified (even if all the data for the "species" are zeroes).
Next we consider the thermodynamical implications of stating reactions such as Equation 4–1
and Equation 4–3
. In the Atomic Site Formalism, the
interpretation is straightforward. In Equation 4–1
we have converted
AsH3 (g) and Ga(s) into AsH3 (s) and Ga(b).
Thus, the change in a thermochemical property, for example, , is the difference in the heats of formation of the products and the
reactants.
For the Open Site Formalism, we need to consider the properties of the open-site species, which may be less easy to define. Since the open-site formalism describes an identical physical event to the atomic-site formalism, the properties of the open site must be related to those of Ga(b) and Ga(s). For example, the heat of formation of this open site is
(4–4) |
Thus, if the thermodynamic properties of Ga(s) were taken to be equivalent to those of Ga(b), then the heat of formation of the open site would be zero. In most cases the thermodynamic behavior of the surface is considered only in relative, not absolute, terms. For this reason, it is important to specify thermodynamic property values of surface sites relative to one species (such as a solid bulk species) in the system and to use this convention, as well as the choice of atomic vs. open-site formalism, throughout the surface reaction mechanism.