Species properies described here include:
The liquid species heat capacity is a required input property and is usually available from established property databases. Chemkin accepts the heat capacity data (coefficients for the curve-fitting equation) from DIPPR and NIST. Table 2.6: Liquid constant-pressure heat capacity models in Chemkin shows how the liquid heat capacity data (from different databases) are specified in the Chemkin input format. The enthalpy and the entropy of a liquid species are derived from the liquid heat capacity and the liquid standard value.
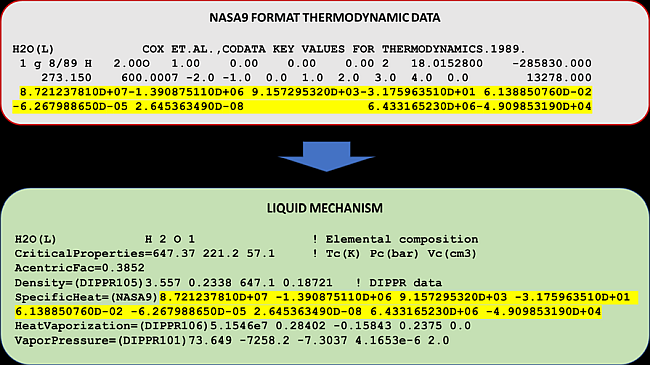
The 9 coefficients of the NASA9 polynomial can be obtained from the thermodynamic data file in the NASA9 format. Most liquid species in the NASA9 thermodynamic data file have only one temperature range so it is straightforward to find the 9 coefficients in the NASA9 data file. Figure 2.1: Finding and entering the coefficients of the NASA9 polynomial for the liquid mechanism shows how the coefficients can be transferred to the liquid mechanism. The NASA 9-coefficient polynomial provides the absolute values of liquid enthalpy and liquid entropy. Therefore, when the NASA 9-coefficient polynomial is specified for the heat capacity, the standard values of liquid enthalpy and liquid entropy are not needed for the species.
Table 2.6: Liquid constant-pressure heat capacity models in Chemkin
| Model Name (units) | Syntax | Equation Form |
| DIPPR100 (J/kmol-K) | specificheat=(DIPPR100) a b c d e | |
| DIPPR114 (J/kmol-K) | specificheat=(DIPPR114) a b c d |
|
| Shomate-NIST(J/mol-K) | specificheat=(SHOMATE) a b c d e |
|
| Constant (J/mol-K) | specificheat=(CONSTANT) | |
| NASA9 (-) | Specificheat=(NASA9) a1 a2 a3 a4 a5 a6 a7 b1 b2 | |
The enthalpy of a liquid species is related to the liquid heat capacity as
(2–130) |
When the liquid is given by DIPPR Equation 100, the liquid enthalpy at temperature
is computed as following
(2–131) |
(2–132) |
When the liquid is given by DIPPR Equation 114, the liquid enthalpy at temperature
is computed as following
(2–133) |
(2–134) |
When the liquid is set to a fixed value, the liquid enthalpy at temperature
is obtained as
(2–135) |
The entropy of liquid species can be derived from its heat capacity as
(2–136) |
When the liquid is given by DIPPR Equation 100, the liquid entropy at temperature
is computed as following
(2–137) |
(2–138) |
When the liquid is given by DIPPR Equation 114, the liquid entropy at temperature
is computed as following
(2–139) |
(2–140) |
When the of a liquid species is set to a fixed value, the liquid entropy at
temperature
is obtained as
(2–141) |