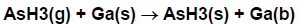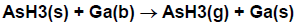In this section we discuss the mathematical formalism developed to describe surface kinetics for events such as adsorption, desorption, surface reactions, and deposition. This formalism is essentially a set of rules for keeping track of surface species concentrations, conservation of mass and surface sites, mass-action kinetics, and rates (such as deposition or etching rates).
For this discussion we define three types of species: gas-phase, surface, and bulk. The first is a species in the gas phase above the surface, which might be denoted in a reaction by (g). A surface species, perhaps denoted by (s), is defined to be the chemical species on the top-most layer of the solid, that is, at the solid-gas interface.
Note: In actuality there is no constraint that the surface must be only one atom thick. However, defining a “surface” that is several monolayers thick may be conceptually much more difficult to deal with.
Each surface species occupies one or more "sites;" the total number of sites is often assumed to be conserved. Any species in the solid below the surface layer is defined to be a "bulk" species and might be denoted by (b). In writing elementary reactions for a surface mechanism in a kinetic model, mass, elemental composition, and charge must all be conserved.
There can be more than one type of site on the surface. For example, one could specify that a surface consists of “ledge” sites and “plane” sites. The number of sites of each type might be a characteristic of the crystal face. In our formalism there can be any number of site types. One may define a species that only resides on a certain type of site. For example, the thermodynamic properties of a hydrogen atom on a ledge site might be different from a hydrogen on a plane site, and they could be specified as different species (even though their elemental composition is the same). The population of different species occupying a given type of site is specified by site fractions. The sum of the site fractions of the species on a given site is 1. (Thus an "open site" is considered as a distinct species.)
In the bulk there can be different types of bulk species. The simplest consists of a pure species. There can be any number of pure bulk species. It is also possible to specify a bulk mixture with components A and B. The composition of the bulk phase may be input by the user by specifying the activities of each of the bulk-phase components.
The activity of a bulk species is defined in terms of the following equation for the chemical potential:
(4–35) |
where is the standard state chemical potential of species
at temperature
and at the standard pressure, 1 atm. The vector
represents an array of the mole fractions of the species. Two conventions are normally used
to complete the specification of the activity coefficient:
If the standard state is defined as a pure bulk phase of
at temperature
and 1 atm, then
is further defined to approach
as
approaches 1 at 1 atm (Raoult's Law).
If the standard state is defined as the hypothetical state of species
in infinite dilution in bulk-phase species
at temperature
and 1 atm, then is further defined to approach
as
approaches 0 at 1 atm (Henry's Law).
Both conventions for the standard state work with the KINetics module. The activities of all components of an ideal solution must sum to 1; this condition is enforced by KINetics.
Since the user may define a number of different types of species (gas species, any number of types of surface sites, species residing on surface sites, pure bulk species, bulk mixtures, and species present in a bulk mixture), it is necessary to track them throughout the simulation. We use the notion of different physical “phases” to group the chemical species in a problem. Our nomenclature corresponds to that of Eriksson [8], which has been extended to account for surface sites. The order in which we discuss the phases is the order in which they are grouped internally in the mechanism data structure.
Phase number 1 is the gas phase. Information about species in the gas phase is extracted from the gas-phase kinetics input. For this phase, the mole fractions of the gas-phase species correspond to species activities, mentioned below.
The next phases that may be included in a surface-chemistry mechanism are surface site phases. We consider
every type of surface site to be a distinct “phase.” If there are types of sites specified, then phases 2 through
are these sites. The user can specify the names of chemical species that exist only
on a given site type. The site fractions of all species that can exist on a given type of site (phase) sum to 1.
The surface species site fractions also correspond to activities.
The last type of phase is a bulk mixture. If a given problem has different types of bulk mixtures, then these are considered to be
phases
through
. The user specifies the names of the species that can exist in a given bulk mixture.
The amounts of these species are determined indirectly by their activities, which the user supplies. A limiting
case is a pure bulk species, which is treated as a bulk mixture with only one chemical species, whose activity
is unity if the chemical potential does not depend on pressure.
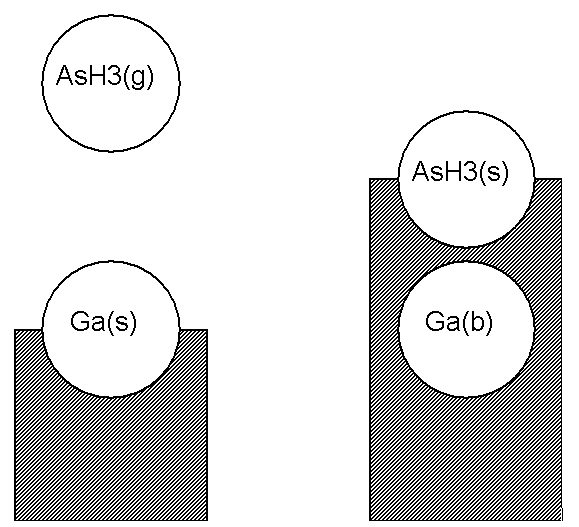
We now consider in more detail how to write chemical reactions involving surface and bulk species. A chemical species on the top layer of the solid, that is, a surface species, occupies a site. For example, an arsine molecule adsorbed on a surface could occupy a site, and might be denoted AsH3(s). Another example might be a bare gallium atom, Ga(s), on top of a gallium arsenide crystal. What happens if another species, say a gas-phase AsH3, lands on top of the Ga(s) (see Figure 4.4: Illustration of an adsorption reaction using the Atomic Site Formalism)? In this case the gallium atom that was at the surface is covered up, so it is no longer a surface species. In our nomenclature it has become a bulk species. The adsorbed AsH3 now occupies the top-most layer at this site, so it has become the surface species AsH3(s). In our formalism, we would write the adsorption reaction in Figure 4.4: Illustration of an adsorption reaction using the Atomic Site Formalism as
In this reaction, the number of sites included on the left-hand side of the reaction equals the number on the right-hand side; the reaction conserves sites.
Suppose that we had wanted to describe the reverse reaction, that is, desorption of AsH3 from the surface. We would then write the reaction as
Here, is included as a reactant in order to achieve site and elemental balance.
We denote the formalism described in Figure 4.1: Example reaction for adsorption, using Atomic Site Formalism and
Figure 4.2: Example reaction for desorption, using Atomic Site Formalism as the Atomic Site Formalism.
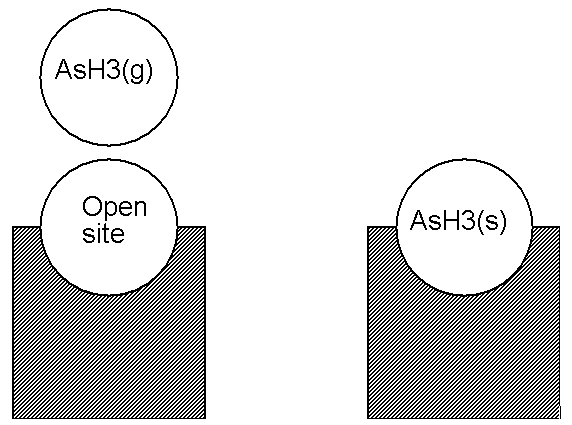
An alternate way of posing the above example is to look at the situation on the left side of Figure 4.4: Illustration of an adsorption reaction using the Atomic Site Formalism not as having a surface gallium atom on a site, but to say that this is really an “open” site at which some event may take place (see Figure 4.5: Illustration of an adsorption reaction using the Open Site Formalism). We would write the reaction of Figure 4.5: Illustration of an adsorption reaction using the Open Site Formalism as
where the symbol O(s) was used to denote an open site. Since O(s) contains no elements (it is empty), this reaction conserves both sites and elements. We denote the formalism described in Figure 4.3: Example reaction for adsorption, using Open Site Formalism as the Open Site Formalism.
The Atomic Site and Open Site formalisms are equally valid ways of stating these surface reactions. Either is allowed by the Surface Kinetics Pre-processor. Personal preference or, perhaps, the nature of a particular problem might dictate one over the other. Note that an “open” site must be considered as a species.
What are the thermochemical implications of reactions such as Figure 4.2: Example reaction for desorption, using Atomic Site Formalism and Figure 4.3: Example reaction for adsorption, using Open Site Formalism? In the
Atomic Site Formalism, the interpretation is straightforward. In Figure 4.2: Example reaction for desorption, using Atomic Site Formalism we have converted
AsH3(g) and Ga(s) into AsH3(s) and Ga(b).
Thus, the change in a thermochemical property, for example, , is just the difference in the heats of formation of the products and the
reactants. How does this compare to the Open Site Formalism? What are the properties of
O(s), the open site? Because these two formalisms describe an identical physical event, it
is evident the properties of the open site must be related to those of Ga(b) and Ga(s).
For example, the heat of formation of this open site is just
(4–36) |