Once you have defined all of the relevant species, you can define the chemical reactions in which they participate. Select the Define reactions menu item in the task menu and follow the steps below for each reaction you want to define.
![]() Define reactions
Define reactions
Specify the equation for the chemical reaction.
 Create a new reaction
Create a new reaction
Ansys Polydata will open a dialog box and request that you enter the reaction equation.
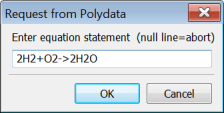
An equation consists of references to species data fields, integers, operators (-> or <=>), and the plus sign +, and has the following form:
where stands for a reactant is a stoichiometric coefficient for a reactant stands for a product is a stoichiometric coefficient for a product There can be up to five reactants and five products in a reaction. Duplicate species are not allowed, and stoichiometric coefficients must be integers with four digits or less. If you dot not specify a coefficient, a value of one will be assumed.
If your reaction is reversible, you can enter it using the <=> operator, or enter it as two separate reactions (the forward and the reverse). Note, however, that entering it as two separate reactions requires more input and uses up two of the ten places in the list of chemical reactions.
Embedded and trailing blanks will be ignored. Checks are performed from left to right. If a parsing error occurs, the formula is printed up to where the parser found the error, and you will see useful error statements like "equation statement is incomplete", and so forth. Use this information to correct syntax errors in the formula.
Once you have entered the equation correctly, the formula will appear in the reaction list. Some examples of valid and invalid equations, using three species with nicknames a1, a2, and a3 is as follows:
valid: 1 a1 + 2 a2 -> 3a3 a1 <=> 2a3 a1 -> 2 a3 2 a3 -> a1 invalid: a1 + 2 -> a3 (missing species identifier) a1 + a2 <=> a1 + a3 (a1 appears twice) a1 ++ a2 -> a3 (adjacent plus signs) a1 + a2 -> a4 (a4 is not pre-defined) Set the parameters for the reaction.
Select the reaction equation in the Define reactions menu.
Check the mass balance.
 Check mass balance
Check mass balance
A nonzero mass balance, while incorrect, will not yield an error message, nor will it cause the chemical reaction to be rejected. You should therefore be sure to check the mass balance yourself.
Specify the domain of the reaction
 Domain of reaction
Domain of reaction
This option allows you to select the subdomain(s) where the chemical reaction will take place. By default, the domain of the reaction is the entire mesh, but restriction to particular subdomains of the mesh is perfectly valid. Chemical reactions cannot be restricted to a boundary or an interface.
If the reaction is reversible and you want to reverse it, select the Reverse reaction menu item.
 Reverse reaction
Reverse reaction
If the rate constant of either the forward or the reverse reaction is known, the reaction speed in the opposite direction can be calculated from the reaction equilibrium thermochemistry. The reaction mechanism for the reversed reaction is the same as for the original, with the products becoming the reactants and vice versa. If you want, you can modify the reaction parameters, using the reversed reaction as a shortcut for defining a new reaction.
Specify the reaction rate constant, by defining the parameters used in Equation 19–11.
 Rate ’constant’
Rate ’constant’
Enter the values for Ta, Tr, prefac (
), beta, Ea/R (
), and entalp (enthalpy used in the energy equation; an exothermic reaction corresponds to a positive value for enthalpy, and an endothermic reaction corresponds to a negative value).
Specify a name for the reaction equation.
 Modification of equation name
Modification of equation name
By default, the equations are given names like forward #1. You can use this menu item to give them more descriptive names for later reference.
If appropriate, switch the reaction orders from integer numbers (the default) to real numbers (optional).
 Switch to real orders
Switch to real orders
Exponential orders are initialized on the basis of the species stoichiometric coefficients: the exponential orders of the direct (or reverse) reaction are assigned the values of the reactants’ (or products’) stoichiometric coefficients; as can be seen, the molar rate of the direct (or reverse) reaction can also depend upon the product (or reactant) species according to the so-called generalized Kamal’s model, which accounts for all species present.
If the orders are integers, then a negative mole concentration will not cause a floating point error. If real orders are selected, then the signed modulus of the mole concentration is used when evaluating the molar rate. For most cases, there will be no reason to change to real orders.
Important: For computational reasons, real orders of reaction between 0 and 1 should be avoided. Also, evolution on the exponential orders, whether integer or real, is not permitted.
(optional) If necessary, modify the values of the exponential powers for the species orders (
in Equation 19–9).
 Modification of power of c(species)
Modification of power of c(species)
By default, the exponential power for a reactant is its stoichiometric coefficient and the exponential power for a product is zero.
Here is an example to illustrate how source terms are distributed among the various species transport equations present. Consider the following chemical system:
| reaction #1: | 2A + 3B | → | 4C |
| reaction #2: | A | → | 2D |
| reaction #3: | 2D | → | A |
| reaction #1: | |
| Source term in transport of A: |
|
| Source term in transport of B: |
|
| Source term in transport of C: |
|
| reaction #2: | |
| Source term in transport of A: |
|
| Source term in transport of D: |
|
| reaction #3: | |
| Source term in transport of A: |
|
| Source term in transport of D: |
|
so that the net mass rate of production/destruction of the various species becomes
In parallel, the net contribution to the energy equation will be given by


