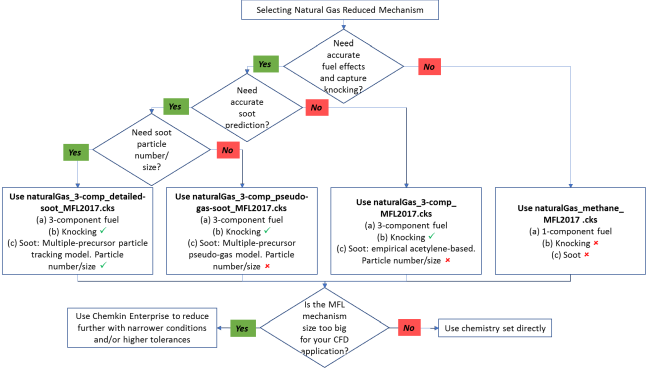
Four natural gas reduced mechanisms are available. The flowchart shown in Figure 2.3: Decision flowchart for selecting MFL natural gas mechanism summarizes the applicability of the four mechanisms. More details of these four mechanisms are presented in the subsections below.
The naturalGas_methane_MFL2017.cks chemistry set represents natural gas with methane (mechanism name: ch4) as a surrogate. The focus of this chemistry set is on modeling combustion at high-temperatures in gas turbines. This chemistry can be used to predict CO, HC (hydrocarbons), and NOx emissions from turbines. The mechanism has been reduced for the following range of conditions:
Pressure: 10–100 bar
Temperature: 1000–2000 K
Equivalence ratio: 0.4–2
EGR: 0–20%
This mechanism has been reduced from the full mechanism “GaseousFuels_C0-C6_NOx” in the MFL database, which has been thoroughly validated against fundamental experimental data for the operating conditions of interest for gas turbines. The mechanism was reduced from this comprehensive full mechanism using the Reaction Workbench software, for the conditions listed above. A high-temperature kinetics filter was used as a first step for mechanism reduction prior to using other reduction methods in Reaction Workbench.
For multi-component natural gas, the surrogate used is 93/ 5/ 2 vol% Methane/ ethane/ n-butane. There are three chemistry sets for natural gas with this 3-component surrogate:
naturalGas_3-comp_MFL2017.cks
naturalGas_3-comp_pseudo-gas-soot_MFL2017.cks
naturalGas_3-comp_detailed-soot_MFL2017.cks
The difference between the three chemistry sets is the soot model they can be used with; they are any acetylene-based empirical soot model, the pseudo-gas soot model, and the Method of Moments soot model, respectively. The chemistry set may be applied to compositions that are different from this, provided that methane is the dominant component. We recommend lumping other C2 content into the fraction represented by ethane and lumping heavier-than-C2 hydrocarbons into the fraction for n-butane. The focus of this chemistry set is on modeling combustion at low- to high-temperatures in gas turbines and on soot emissions. It can be used to track soot particle mass, number and size in Ansys Chemkin and using the Method of Moments in Ansys Forte.
The mechanism has been reduced for the following range of conditions:
Pressure: 10–100 bar
Temperature: 800–2000 K
Equivalence ratio: 0.4–3
EGR: 0–20%
This mechanism has been reduced from the full mechanism “Gasoline_PAH_NOx” in the MFL database, which has been thoroughly validated against fundamental experimental data for the operating conditions of interest for gas turbines. The mechanism was reduced from this comprehensive full mechanism using the Reaction Workbench software, for the conditions listed above.
For the emissions, the following species predictions are expected to be accurately predicted:
Soot-precursor species:
acetylene (c2h2)
butadiyne (c4h2)
propargyl (c3h3)
benzene (c6h6)
toluene (c6h5ch3)
acenaphthalene (a2r5)
pyrene (a4)
coronene (coronene)
CO (co)
NOx (no and no2)
Unburned hydrocarbons
The species names in the chemistry file for the fuel species are:
Methane is ch4.
Ethane is c2h6.
n-Butane is c4h10.