After enabling beta features as described in Introduction, you can account for van der Waals forces in 3D cases that involve discrete element method (DEM). Including van der Waals forces in DEM simulations is most important for particle diameters smaller than 40 to 100 micron.
Note: This capability is not supported in 2D.
Based on the studies of London [3] and
Hamaker [1], the van der Waals interaction
potential for two spherical particles, , can be calculated
by [2]:
(14–1) |
| where: | |
|
| |
|
| |
|
|
can be calculated by:
where and
are the Hamaker constants of the materials of the two particles,
respectively.
The van der Waals force acting on a particle located at by another particle located at
is calculated by:
(14–2) |
This is a force of attraction with the magnitude of the partial
derivative of with respect to
that points from the center
of the first particle to the center of the second particle,
. For particle-wall interaction,
the van der Waals force points in the direction of the shortest distance
between the particle and the wall.
To avoid a singularity problem in Equation 14–2 when the particle surfaces are in contact (that is, =0), the van der Waals force
of attraction is clipped, that is it stops increasing, as the particle
separation drops below a user-specified value,
force-limiting-distance.
To model van der Waals forces during DEM particle-particle or particle-wall interactions:
In the DEM Collision Settings dialog box (that opens by clicking Set... in the DEM Collisions dialog box), from the Van Der Waals drop-down list, select
van-der-waals-hamaker.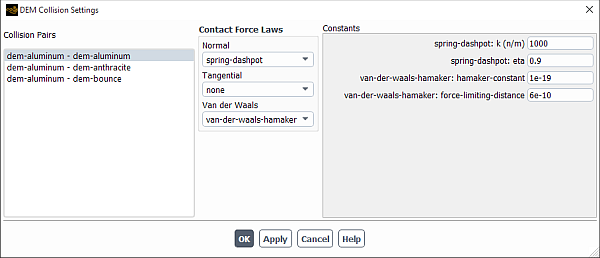
Specify the following force law parameters:
hamaker-constant:in Equation 14–1. Default value: 1e-19 J.
force-limiting-distance: is used to define an upper limit for the van der Waals force. Default value: 6e-10 m.


