In the evaluation of a mechanism, the Reaction Path Analyzer provides graphical descriptions of the pathways of formation in a mechanism. A common example of the use of reaction pathway analysis is in studying the decomposition in a methane air flame. To verify the Reaction Path Analyzer against such a benchmark, then, a methane combustion mechanism [2] was investigated with assistance from the Reaction Path Diagram. A Burner Stabilized Flame simulation was performed with Chemkin using the USC mechanism from Wang [2]. Conditions are: atmospheric pressure, 300-K inlet temperature, and a methane-air equivalence ratio of one. From previous studies, it is expected that depletion of methane species in the pre-flame region occurs primarily through hydrogen abstraction by hydroxy radical. Later in the warmer region of the flame other hydrogen abstraction reactions begin to match the magnitude of the hydroxyl radical hydrogen abstraction reaction.
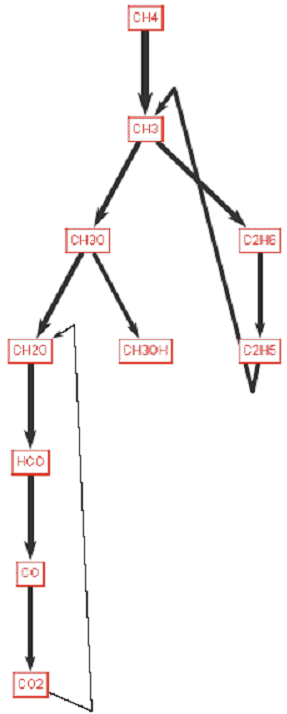
A Reaction Path Diagram for this case is shown in Figure 4.13: Methyl decomposition pathways. The diagram is displayed at a temperature of 1400 K. Analysis of the diagram indicates that the main pathways of decomposition of methyl radical is to the formation of the excited CH2 species, which then decomposes to form CH2 O and CH3 O. Other channels lead directly from methyl radical to the radical-radical recombination to ethane. The primary depletion of ethane is through the hydrogen abstraction to ethyl radical, which through radical-radical recombination with a methyl radical leads to the formation of propane. In the hotter region of the flame, these molecular-weight growth reactions are less favored. All of this is consistent with previous, published findings.