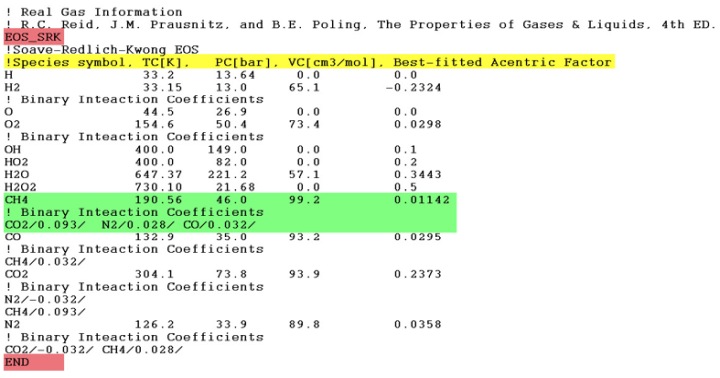
Ansys Chemkin employs the cubic equation of state (EOS) to capture the P-T-V relationship of a
real gas mixture at high pressures. For each gas species in the real gas mixture, the cubic
equation of state models require additional properties that are not part of the regular
thermodynamic data. These properties include the critical point, ,
, and
, and the acentric factor ω, and would be provided in the real gas data
block of the gas-phase mechanism. The real gas data block starts with the keyword EOS_.
Immediately following this keyword (no space), a phrase indicating the choice of the cubic
equation of state model to be used with this gas mechanism is appended. There are five cubic EOS
models available in Ansys Chemkin. (For more details, see Real Gas Model in the Chemkin Theory Manual). These cubic
equation of state models, along with their key phrases, are listed in Table 3.3: Cubic equation of state models available in Chemkin. For example,
the keyword EOS_PR will cause the Peng-Robinson equation of state to be applied whenever this gas
mechanism is used in a Ansys Chemkin simulation.
Table 3.3: Cubic equation of state models available in Chemkin
| Cubic EOS Model | Key Phrase |
|---|---|
| van der Waals | VAND or VDW |
| Redlich-Kwong | REDL or RK |
| Soave-Redlich-Kwong | SOAV or SRK |
| Aungier-Redlich-Kwong | AUNG or ARK |
| Peng-Robinson | PENG or PR |
The required properties of all gas species in the mechanism are given below the EOS_
keyword. Each gas species will have its properties listed in one single line. The line must start
with the species name and be followed by, in the exact same order, the values of ,
,
, and ωω for this gas species. The critical pressure
is in bar, the critical temperature
in Kelvin, and the critical molar volume
in cm3/mole. All four parameters are required. If
or ω are not available for a species, set the value to zero.
The parameters are format-free and separated by blank space(s). If the binary interaction coefficients between this gas species and other species are known, the coefficients can be given as auxiliary keywords in the line(s) below the property data line. The interacting species name and the corresponding binary interaction coefficient should be provided in the format illustrated here with the coefficient value delimited by slashes (/):
<interacting species name>/<interaction coefficient>/
For example, the binary interaction coefficient between O2 and N2 can be given in a line
below the properties data line of O2 (or N2) as N2/-0.0078/ (or
O2/-0.0078/).
If you have multiple binary interaction coefficients to specify, they should be separated by blank space(s) and can be written in a single line or in several lines. The binary interaction coefficient is only required once for each pair of species.
All species in the mechanism must have their real gas data given in the real gas data block,
and the species data lines can appear in any order. The real gas data block should be closed by
the keyword END.
Figure 3.3: Example of Real Gas data input shows some examples of the real gas data input.