We illustrate the use of Surface Kinetics by a simple example involving deposition of silicon. The surface reaction mechanism is shown in Example 1.5: Sample Reaction Mechanism as Read by the Surface
Kinetics Pre-processor
as it appears for the input file to the Surface Kinetics Pre-processor. The first two lines identify a site type called SILICON that has a site density of 1.66 × 10-9 mole/cm2. Only one species, SI(S), exists on this site type. The bulk material is identified as SI(B), and it has a mass bulk density of 2.33 g/cm3. This is a very simple example that has only one site type occupied by only one species and only one pure bulk material. In general, however, an input file could specify many different site types, each of which could be occupied by a variety of species. Furthermore, there could be several bulk-phase mixtures that could each be composed of several species. Examples of all these possibilities appear in the Chemkin Input Manual
Input Manual.
The reaction mechanism itself is listed next. The symbol => in each reaction expression indicates that all of the reactions are irreversible. The three numbers following each reaction expression are its Arrhenius rate parameters ( pre-exponential factor, temperature exponent, and activation
energy).
Example 1.5: Sample Reaction Mechanism as Read by the Surface Kinetics Pre-processor
SITE/SILICON/ SDEN/1.66E-09/ SI(S) BULK SI(B) /2.33/ REACTIONS SIH4 + SI(S) =>SI(S) + SI(B) + 2H2 1.05E17 0.5 40000 SI2H6 +2SI(S) =>2SI(S) + 2SI(B) + 3H2 4.55E26 0.5 40000 SIH2 + SI(S) =>SI(S) + SI(B) + H2 3.9933E11 0.5 0 SI2H2 +2SI(S) => 2SI(S) + 2SI(B) + H2 1.7299E20 0.5 0 2SI2H3 +4SI(S) => 4SI(S) + 4SI(B) + 3H2 6.2219E37 0.5 0 H2SISIH2 +2SI(S) => 2SI(S) + 2SI(B) + 2H2 1.7007E20 0.5 0 2SI2H5 +4SI(S)=> 4SI(S) + 4SI(B) + 5H2 6.1186E37 0.5 0 2SIH3 +2SI(S) => 2SI(S) + 2SI(B) + 3H2 2.3659E20 0.5 0 2SIH +2SI(S) => 2SI(S) + 2SI(B) + H2 2.4465E20 0.5 0 SI + SI(S) => SI(S) + SI(B) 4.1341E11 0.5 0 H3SISIH +2SI(S) => 2SI(S) + 2SI(B) + 2H2 1.7007E20 0.5 0 SI2 +2SI(S) => 2SI(S) + 2SI(B) 1.7607E20 0.5 0 SI3 +3SI(S) => 3SI(S) + 3SI(B) 8.6586E28 0.5 0 END
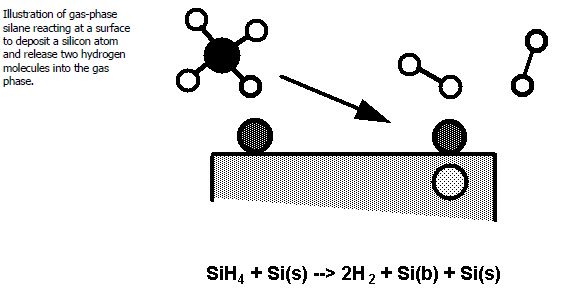
All of the reactions in this mechanism have the same form: a gas-phase species reacting on a silicon site. The reaction of silane at the surface is illustrated in Figure 1.4: Illustration of Gas-Phase Silane Reaction . Each silicon-containing gas-phase species can react on an atomic surface site, SI(S), to deposit a silicon atom as SI(B) and release hydrogen back into the gas phase. We have included SI(S) as both a reactant and a product to indicate that a "site" must be available at which the gas-phase species can react. In the example, however, the surface silicon SI(S) is distinguished from the bulk deposit SI(B) by virtue of its position as the top-most atom at the surface. Therefore, each time a SI(S) is consumed by a reaction the bulk layer becomes one atom thicker and the silicon atom that just left the gas now forms the top-most surface layer, that is, SI(S). For this mechanism, the SI(S) could have been just as well left out of the mechanism entirely. However, if other gas-phase species had been present (say phosphine carrying phosphorus as a dopant), these species could compete for the available silicon sites on the surface. Thus, by writing the reactions as we have, we have left open the possibility for other species to occupy surface sites and thus inhibit the deposition of silicon.
As an example of the full use of Surface Kinetics, assume that the program we are writing needs to evaluate a boundary condition concerning the energy balance at a surface of an isothermal particle.
The energy balance would take the following form (with the surface normal pointing into
the particle):
(1–3) |
The dependent variables in this expression are the temperature , gas-phase mass fractions
and convective velocity
. The surface site fractions and the bulk-species activities are also dependent variables, but do not appear explicitly in the expression. The first term in this equation describes thermal conduction to the surface from the gas phase. The thermal conductivity
would be evaluated by a call to the Transport
Subroutine Library, and the temperature gradient could be evaluated by finite differences. The second term concerns the diffusive and convective flux of energy by gas-phase species at the surface. The mass density
and the gas-phase enthalpies
would be evaluated by calls to the Gas-phase Kinetics Subroutine Library. The gas-phase species diffusion velocities
would be evaluated in terms of diffusion coefficients that are obtained from the Transport Package and finite difference approximations to the species gradients. The first term on the right-hand side concerns the thermal radiation to or from the surface.
We now concentrate on the final term, which concerns the energy generated or consumed from surface reaction. The summation is over all surface and bulk species, and the factors in the summation are the production rate of surface and bulk species by surface reaction, , the species molecular weights,
, and the enthalpies of the surface and bulk species,
. The FORTRAN representation of this term begins with Surface Kinetics Subroutine Library subroutine calls (the output variables are underlined to help distinguish them):
Example 1.6: Example calls to the Surface Kinetics Subroutine Library
CALL SKINIT(LSIWK, LSRWK, LSCWK, LINKSK, LOUT, ISKWRK, RSKWRK, CSKWRK, IFLAG) CALL SKWT(ISKWRK, RSKWRK, WT) CALL SKHMS(T, ISKWRK, RSKWRK, HMS) CALL SKRAT(P, T, ACT, SDEN, ISKWRK, RSKWRK, SDOT, SITDOT)
The complete details for these calls are explained in later chapters of this manual; the
objective here is to illustrate the relative simplicity of writing an
Ansys Chemkin program. Briefly, the first call is to the
initialization subroutine SKINIT, which reads the
Surface Kinetics Linking File created by the
Surface Kinetics Pre-processor and populates the three work
arrays. LSIWK, LSRWK, and
LSCWK are the dimensions provided by the user for the data arrays
ISKWRK, RSKWRK, and CSKWRK.
LINKSK is the logical file number of the Surface
Kinetics Linking File, LOUT is the logical file
number for printed diagnostic and error messages, and IFLAG is an
integer error
flag. In the remaining calls, P and T are the
pressure and temperature. The array ACT
contains the
gas-phase mole fractions, the
surface site
fractions, and the
bulk species activities, in that order. The output
variable arrays, HMS and SDOT, correspond to the
factors in the summation from Equation 1–3
, that is, HMS =
, and
SDOT = .
The FORTRAN representation of the summation in the last term, given by combining the results of the above subroutine calls, is simply
Example 1.7: Example FORTRAN code for calculating equation terms
SUM=0.0
DO 100 K=FIRST_SURFACE_SPECIES, LAST_BULK_SPECIES
SUM = SUM + SDOT(K)*WT(K)*HMS(K)
100 CONTINUEThe species indices FIRST_SURFACE_SPECIES and LAST_BULK_SPECIES are also available from a call to the Surface Kinetics Subroutine Library.