Referring to the unburnt mixture, the equivalence ratio describes
the ratio of fuel relative to the amount of fuel that potentially
could be burnt with the available oxidizer. For stoichiometric mixture,
the equivalence ratio is defined to be
, that is, the amount of
fuel and oxidizer match such that they could be burnt with neither
fuel nor oxidizer left behind.
indicates fuel-rich mixtures (excess fuel),
and
indicates fuel-lean mixtures (excess oxidizer).
The limits are
for pure oxidizer and
(infinite)
for pure fuel.
When the stoichiometric mixture fraction is known, the local equivalence ratio can be computed
from mixture fraction
according to
(7–49) |
The stoichiometric mixture fraction depends on the fuel and the oxygen content in the
oxidizer and is a property of the flamelet library.
Value
This option enables you to specify directly the stoichiometric mixture fraction used for calculating the equivalence ratio.
Reactants
For this option, the reactants and their stoichiometric coefficients for a representative global reaction are specified. For example, for a single component fuel,
(7–50)
or for the generic form,
(7–51)
The stoichiometric mixture fraction is computed using the reactants stoichiometric coefficients and the corresponding species mass fractions in the fuel and oxidizer streams, respectively. The species mass fractions in the fuel and in the oxidizer are obtained from the flamelet library.
Automatic
This option derives the stoichiometric mixture fraction from the flamelet library requiring no additional information. The numerical procedure is described below.
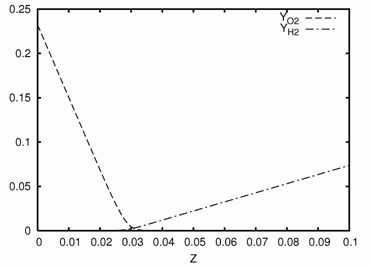
Figure 1 shows the qualitative behavior of mass fractions for fuel and oxygen plotted over mixture fraction (hydrogen/air in the example).
One can observe that the oxygen concentration is approximately linear below and above the stoichiometric mixture fraction: linear decay on the lean side and constantly zero on the fuel side. Obviously, the curvature of the curve is close to zero except near the sharp bend at stoichiometric mixture fraction. This observation is generalized to establish the following procedure
The stoichiometric mixture fraction approximated by the point
of maximum curvature for oxygen mass fraction, or , is
(7–52) |
This is a heuristic approach and only provides an approximation. You should check the plausibility of the calculated value, which is reported to the CFX-Solver Output file. For details, see CFX-Solver Output File (Combustion Runs) in the CFX-Solver Manager User's Guide.